1,11-十二二炔 | 20521-44-2
中文名称
1,11-十二二炔
中文别名
——
英文名称
1,11-dodecadiyne
英文别名
dodeca-1,11-diyne
CAS
20521-44-2
化学式
C12H18
mdl
——
分子量
162.275
InChiKey
DVVJEEGECYXPEC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:99°C 15mm
计算性质
-
辛醇/水分配系数(LogP):4.6
-
重原子数:12
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
反应信息
-
作为反应物:描述:1,11-十二二炔 在 [(η5-C5Me5)RuCl(μ2-SMe)2Ru(η5-C5Me5)Cl] ammonium tetrafluoroborate 作用下, 以 甲醇 为溶剂, 反应 1.0h, 以48%的产率得到(Z)-1-Cyclododecen-3-yne参考文献:名称:硫醇盐桥联二钌配合物催化的末端二炔环化:内大环 (Z)-1-En-3-ynes 的简单合成路线摘要:DOI:10.1002/1521-3773(20000818)39:16<2909::aid-anie2909>3.0.co;2-r
-
作为产物:参考文献:名称:费歇尔卡宾配合物的分子内环己二烯酮环化:合成泛素的模型研究。摘要:铬卡宾配合物的分子内环己二烯酮环化已作为一种方法提供了普遍使用的天然产物Phomactin系列的方法。卡宾配合物的立体化学的重要性和连接卡宾配合物和炔烃的系链中碳的数目进行了探讨。另外,检查了1,4-不对称诱导的程度。DOI:10.1021/ol070904q
文献信息
-
Syntheses and solid state structures of cyclic diynes with two chalcogen centres ? a competition between weak interactions作者:J. Hilko Schulte、Daniel B. Werz、Frank Rominger、Rolf GleiterDOI:10.1039/b303653g日期:——Structural investigations by means of X-ray diffraction reveal for most systems a chair-like conformation in the solid state. For 5S(2)2, 5S(2)3, 7S(2)5, 5Se(2)2 and 5Te(2)3 tubular structures were encountered. These structures can be traced back to weak XX or weak C-Hpi interactions.在本文中,我们报告了通式为mX2n的环状二炔的合成。字母m和n表示两个C三键CX单元之间的链长,其中X表示S,Se或Te。桥的长度在m = 4-8和n = 2-6之间变化。通过X射线衍射进行的结构研究表明,对于大多数系统而言,固态呈椅子状构象。对于5S(2)2、5S(2)3、7S(2)5、5Se(2)2和5Te(2)3,遇到管状结构。这些结构可以追溯到弱XX或弱C-Hpi相互作用。
-
Cyclic Diynes by Alkyne Metathesis作者:Rolf Gleiter、Björn Hellbach、Frank RomingerDOI:10.1055/s-2003-42421日期:——termini (lla-i) is described. The methylene groups between the alkyne units vary between n = 12 (a) and n = 4 (i). Ring closing metathesis with Mo(CO) 6 /CF 3 C 6 H 4 OHyielded the monocyclic alkyne 12a with 11a as starting material, whereas 11b-g yielded the cyclic diynes 13b-g. Detailed structural parameters were obtained for 13b and 13c by X-ray crystallography.
-
Alkynol natural products target ALDH2 in cancer cells by irreversible binding to the active site作者:Wolfgang Heydenreuter、Elena Kunold、Stephan A. SieberDOI:10.1039/c5cc06424d日期:——
Chemical proteomic studies reveal ALDH2 as a molecular target of falcarinol in cancer cells.
化学蛋白质组学研究揭示了ALDH2在癌细胞中作为falcarinol的分子靶点。 -
Total synthesis of two novel brominated acetylenic diols (+)-diplyne C and E: stereoselective construction of the (E)-1-bromo-1-alkene作者:Benjamin W. Gung、Craig Gibeau、Amanda JonesDOI:10.1016/j.tetasy.2005.08.019日期:2005.9The total syntheses of the enantiomers of two novel brominated polyacetylenic natural products diplynes C and E are reported. Pd and Cu(I)-catalyzed coupling reactions were employed to synthesize the diyne and enyne units. The stereochemistry of the terminal (E)-alkenyl bromide in diplyne C was constructed stereoselectively using Brown’s hydroboration–bromination procedure. The stereochemistry of the
-
Chemo- and Regioselective Intermolecular Cyclotrimerization of Terminal Alkynes Catalyzed by Cationic Rhodium(I)/Modified BINAP Complexes: Application to One-Step Synthesis of Paracyclophanes作者:Ken Tanaka、Kazuki Toyoda、Azusa Wada、Kaori Shirasaka、Masao HiranoDOI:10.1002/chem.200401017日期:2005.2.4the cationic rhodium(I)/DTBM-Segphos complex. This method can be applied to a variety of terminal alkynes to provide 1,2,4-trisubstituted benzenes in high yield and with high regioselectivity. A chemo- and regioselective intermolecular crossed-cyclotrimerization of dialkyl acetylenedicarboxylates with a variety of terminal alkynes has also been developed based on the use of the cationic rhodium(I)/H8-BINAP基于阳离子铑(I)/ DTBM-Segphos配合物的使用,已开发出高度炔基选择性的末端炔烃分子间环三聚体。该方法可用于各种末端炔烃,以高产率和高区域选择性提供1,2,4-三取代的苯。基于阳离子铑(I)/ H8-BINAP络合物的使用,还开发了乙炔基二羧酸二烷基酯与各种末端炔烃的化学和区域选择性分子间交叉环三聚,可高产率提供3,6-二取代邻苯二甲酸酯。就催化活性,化学和区域选择性,底物范围和易于操作而言,它构成了一种高效的新方法,用于两种不同单炔的分子间交叉环三聚。通过将其应用于从相应的末端α,ω-二炔类化合物中一步合成[6]甲基环已烷和[7]-[12]对环已烯,证明了这种新的交叉炔烃环三聚方法的多功能性。机理研究表明,这种交叉炔烃环三聚的化学和区域选择性是由优先形成的衍生自末端炔烃和乙炔二羧酸二烷基酯的特定金属铑金属环决定的。
表征谱图
-
氢谱1HNMR
-
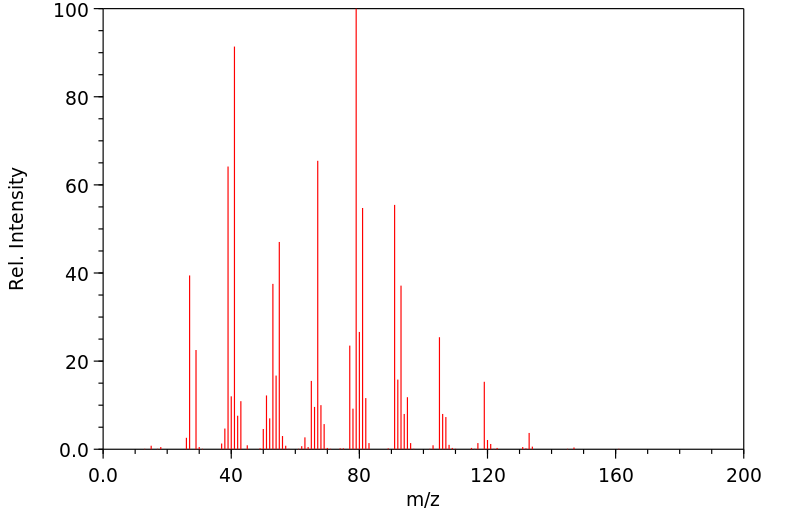
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
锗烷,三甲基[3-(三甲基甲锡烷基)-2-炔丙基]-
锗烷,三甲基-2-炔丙基-
铜,1-戊炔基-
甲基炔丙基硫化物
甲基乙炔和丙二烯混合物
甲基丙-2-炔基氰基二硫代亚氨酸酯
甲基-D3-乙炔
环戊基乙炔
环己基乙炔
环丙乙炔
炔丙胺
炔丙基膦
炔丙基碘化物
炔丙基叔丁基二甲基硅烷
炔丙基三甲基硅烷
炔丙基三乙基硅烷
氘乙炔
戊-1-炔-3-胺
戊-1,3-二炔
戊-1,2-二烯-4-炔
异氰基-乙炔
己基(己-5-炔基)甲基硅烷
己-1-炔银
四碳化铀
反式-4-(2-丙炔基)-环己烷甲醇
双(三甲基锡)乙炔
双(三氟甲基)锌
十四碳-1,4-二炔
十四碳-1,3-二炔
十八碳-1,17-二炔
十八炔
十三碳-1,7-二炔
十三碳-1,12-二炔
十一碳-1,5-二炔
亚硫酸二(2-丙炔基)酯
二甲基炔丙基溴化硫
二炔丙基硫醚
二乙炔基-二甲基-锗烷
二丙-1-炔基汞
二[2-甲氧基乙基汞(II)]乙炔
二(三正丁基甲锡烷基)乙炔
二(3-羟基-1-丙炔基)汞(II)
乙炔锂乙二胺配合物
乙炔银
乙炔基环己烷钠
乙炔基环丙烷氯化镁
乙炔基(三甲基)锗烷
乙炔基(三甲基)硅烷铜(1+)
乙炔基(三甲基)硅烷溴化镁
乙炔基(三甲基)硅烷氯化镁