1,3-二烯丙基脲 | 1801-72-5
中文名称
1,3-二烯丙基脲
中文别名
——
英文名称
1,3-diallylurea
英文别名
diallylurea;N,N'-diallylurea;N,N'-Diallyl-harnstoff;1,3-di-(2-propenyl)-urea;1,3-bis(prop-2-enyl)urea
CAS
1801-72-5
化学式
C7H12N2O
mdl
MFCD00008639
分子量
140.185
InChiKey
QRWVOJLTHSRPOA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:90-93 °C(lit.)
-
沸点:56°C/10mmHg(lit.)
-
密度:1.0897 (rough estimate)
-
溶解度:溶于甲醇
-
稳定性/保质期:
在常温常压下保持稳定,应避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:10
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.285
-
拓扑面积:41.1
-
氢给体数:2
-
氢受体数:1
安全信息
-
WGK Germany:3
-
海关编码:2924199090
-
安全说明:S24/25
-
储存条件:密封储存,应存放在阴凉干燥的库房中。
SDS
| Name: | 1 3-Diallylurea 99% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 1801-72-5 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1801-72-5 | 1,3-Diallylurea | 99% | 217-291-7 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam. Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1801-72-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystals
Color: white - waxy
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 90.00 - 93.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H12N2O
Molecular Weight: 140.18
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1801-72-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1,3-Diallylurea - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1801-72-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1801-72-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1801-72-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,3,4,7-tetrahydro-2H-1,3-diazepin-2-one 72331-40-9 C5H8N2O 112.131
反应信息
-
作为反应物:参考文献:名称:Extension of the Bambus[n]uril Family: Microwave Synthesis and Reactivity of Allylbambus[n]urils摘要:Microwave irradiations allow the preparation of unsaturated bambusurils in 85% yield compared to 20% yield under classical reaction conditions. Five new bambusurils were synthesized including unsaturated derivatives Allyl(8)BU[4] and Allyl(12)BU[6] bearing diallylglycoluril units. The reactivity of Allyl(8)BU[4] was tested in a variety of organic reactions showing that this macrocycle acts as a classical double bond-bearing product. The first monofunctionalized bambusuril Allyl(7)HepBU[4] prepared by a cross metathesis reaction is also reported.DOI:10.1021/ol303277u
-
作为产物:参考文献:名称:Rundqvist, Archiv der Pharmazie, 1898, vol. 236, p. 469摘要:DOI:
文献信息
-
[EN] POLYCATIONIC AMPHIPHILES AND POLYMERS THEREOF AS ANTIMICROBIAL AGENTS AND METHODS USING SAME<br/>[FR] COMPOSÉS AMPHIPHILES POLYCATIONIQUES ET LEURS POLYMÈRES UTILISABLES EN TANT QU'AGENTS ANTIMICROBIENS ET LEURS PROCÉDÉS D'UTILISATION申请人:TEMPLE UNIVERSITY-OF THE COMMONWEALTH SYSTEM OF HIGHER EDUCATION公开号:WO2016172436A1公开(公告)日:2016-10-27The present invention includes novel polycationic amphiphilic compounds useful as antimicrobial agents. The present invention further includes novel polymers of polycationic amphiphilic compounds useful as antimicrobial agents. The present invention further includes methods useful for removing microorganisms and/or biofilm-embedded microorganisms from a surface. The present invention further includes compositions and methods useful for preventing or reducing the growth or proliferation of microorganisms and/or biofilm-embedded microorganisms on a surface.
-
[EN] FUSED PYRIMIDINE-DIONE DERIVATIVES AS TRPA1 MODULATORS<br/>[FR] DÉRIVÉS DE PYRIMIDINEDIONES FUSIONNÉS UTILISÉS COMME MODULATEURS DES RÉCEPTEURS TRPA1申请人:GLENMARK PHARMACEUTICALS SA公开号:WO2010109287A1公开(公告)日:2010-09-30The invention described herein relates to novel fused pyrimidinediones derivatives of formula (I) which are TRPA (Transient Receptor Potential subfamily A) modulators. In particular, compounds described herein are useful for treating or preventing diseases, conditions and/or disorders modulated by TRPAl (Transient Receptor Potential subfamily A, member 1). This invention also provides processes for preparing compounds described herein, intermediates used in their synthesis, pharmaceutical compositions thereof, and methods for treating or preventing diseases, conditions and/or disorders modulated by TRPAl. Formula (I)
-
Modified amino acids, pharmaceuticals containing these compounds and method for their production申请人:——公开号:US20010036946A1公开(公告)日:2001-11-01The present invention relates to modified amino acids of general formula 1 wherein A, Z, X, n, m, R, R 2 , R 3 , R 4 and R 11 are defined as in claims 1 to 5 , their tautomers, their diastereomers, their enantiomers, the mixtures thereof and the salts thereof, particularly the physiologically acceptable salts thereof with inorganic or organic acids or bases, pharmaceutical compositions containing these compounds, the use thereof and processes for preparing them as well as their use for the production and purification of antibodies and as labelled compounds in RIA- and ELISA assays and as diagnostic or analytical aids in neurotransmitter research.
-
NOVEL QUINOXALINEDIONE DERIVATIVES, THEIR PREPARATION AND USE申请人:——公开号:US20030114422A1公开(公告)日:2003-06-19A compound having the formula 1 or a pharmaceutically acceptable salt thereof wherein R is hydrogen or hydroxy; R 1 is hydrogen, alkyl, arylalkyl, (CH 2 ) n OH, or (CH 2 ) NR 7 R 8 ; R 5 and R 6 are each independently hydrogen, halogen, NO 2 , CN, CF 3 , SO 2 NR 7 R 8 , PO 3 R 9 R 10 , alkyl, alkenyl, alkynyl, (CH 2 ) n CONR 7 R 8 , (CH 2 ) n CO 2 R 10 , NHCOR 11 , wherein R 7 and R 8 are each independently hydrogen or alkyl or together R 7 and R 8 form a ring of from three to seven atoms, R 9 is hydrogen or alkyl, R 10 is hydrogen or alkyl, R 11 is hydrogen or alkyl, and n is an integer of from zero to four; A is a ring of five to seven atoms fused with the benzo ring at the positions marked a and b, and formed by the following bivalent radicals: a-NR 12 —CHR 13 —CHR 14 -b, a-CHR 13 —CHR 14 —NR 12 -b, a-CHR 13 —NR 12 —CHR 14 -b, a-CHR 14 —CH 2 —NR 12 —CHR 13 -b, a-CHR 13 —NR 12 —CH 2 —CHR 14 -b, a-CH 2 —CH 2 —CHR 13 —NR 12 -b, a-NR 12 —CHR 12 —CHR 13 —CH 2 —CH 2 -b, a-CH 2 —CH 2 —NR 12 —CH 2 —CH 2 -b, a-CH 2 —CH 2 —CH 2 NR 12 —CH 2 -b, a-CH 2 —NR 12 —CH 2 —CH 2 -b a-CH 2 —CH 2 —CH 2 —CH 2 —NR 12 -b, a-NR 12 —CH 2 —CH 2 —CH 2 —CH 2 -b, wherein R 12 is hydrogen, CH 2 CH 2 OH, or alkyl, and R 13 and R 14 are each independently hydrogen, CN, CONH 2 , CH 2 NH 2 , CH 2 OH, alkyl, arylalkyl, alkenyl, or CO 2 R 15 , wherein R 15 is hydrogen or alkyl. The compounds are useful in the treatment of disorders of mammals, responsive to the blockade of glutamic and aspartic acid receptors. Processes for preparing the compounds and novel intermediate useful in the processes are also included.具有以下式1的化合物或其药学上可接受的盐,其中R为氢或羟基;R1为氢、烷基、芳基烷基、(CH2)nOH或( )NR7R8;R5和R6各自独立地为氢、卤素、NO2、CN、CF3、SO2NR7R8、PO3R9R10、烷基、烯基、炔基、( )nCONR7R8、( )nCO2R10、NHCOR11,其中R7和R8各自独立地为氢或烷基,或者一起形成由三至七个原子组成的环的R7和R8;R9为氢或烷基,R10为氢或烷基,R11为氢或烷基,n为从零到四的整数;A为与苯环在标记为a和b的位置融合的五至七个原子组成的环,并由以下二价基团形成:a-NR12—CHR13—CHR14-b,a-CHR13—CHR14—NR12-b,a-CHR13—NR12—CHR14-b,a-CHR14— —NR12—CHR13-b,a-CHR13—NR12— —CHR14-b,a- — —CHR13—NR12-b,a-NR12—CHR12—CHR13— — -b,a- — —NR12— — -b,a- — — NR12— -b,a- —NR12— — -b,a- — — — —NR12-b,a-NR12— — — — -b,其中R12为氢、 OH或烷基,R13和R14各自独立地为氢、CN、CONH2、 NH2、 OH、烷基、芳基烷基、烯基或CO2R15,其中R15为氢或烷基。这些化合物在治疗对谷氨酸和天冬氨酸受体阻滞有反应的哺乳动物的疾病中有用。还包括用于制备这些化合物的方法和在这些方法中有用的新中间体。
-
Preparation of Mono-, Di-, and Trisubstituted Ureas by Carbonylation of Aliphatic Amines with <b><i>S</i></b>,<b><i>S</i></b>-Dimethyl Dithiocarbonate作者:Rita Fochi、Emma Artuso、Iacopo Degani、Claudio MagistrisDOI:10.1055/s-2007-990813日期:2007.11General procedures are reported to prepare N-alkylureas, N,N'-dialkylureas (both symmetrical and unsymmetrical), and N,N,N'-trialkylureas by carbonylation of aliphatic amines, employing S,S-dimethyl dithiocarbonate (DMDTC) as a phosgene substitute. All reactions were carried out in water. Symmetrical disubstituted ureas were prepared directly working at 60 °C with a molar ratio of DMDTC:amine = 1:2据报道,通过脂肪胺的羰基化制备 N-烷基脲、N,N'-二烷基脲(对称和不对称)和 N,N,N'-三烷基脲的一般程序,采用 S,S-二硫代碳酸二甲酯 (DMDTC) 作为光气替代品。所有反应均在水中进行。在 60°C 下直接制备对称二取代脲,DMDTC: 胺的摩尔比 = 1:2,优选在氮气下。通过在室温下在第一步中选择性形成的 S-甲基 N-烷基-硫代氨基甲酸酯中间体分两步制备不对称脲。这些中间体在第二步中与氨或各种脂肪胺(伯胺和仲胺)在 50 至 70°C 的温度下发生反应。所有目标尿素均以高产率获得(28 个示例,平均收率 94%)和非常高的纯度(通常 >99.2%)。还需要注意的是回收工业利益的副产品甲硫醇,每摩尔 DMDTC 回收量为两摩尔,同时完全利用试剂。
表征谱图
-
氢谱1HNMR
-
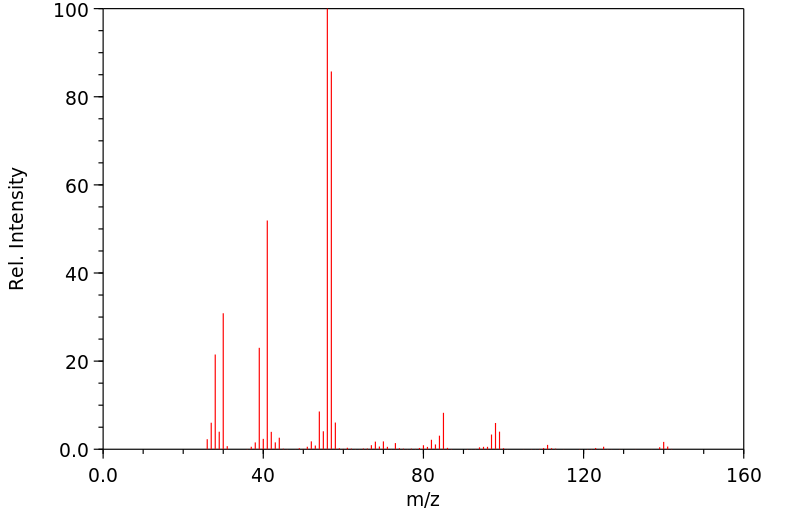
质谱MS
-
碳谱13CNMR
-
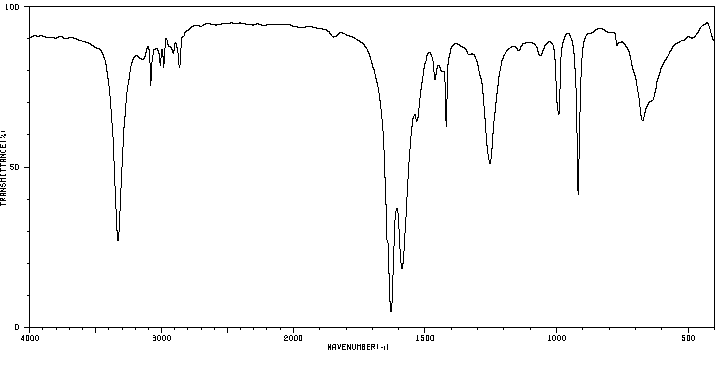
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-4-[(甲基氨基甲酰)氨基]环己烷羧酸
顺式-3-己烯醇碳酸甲酯
镏碳酸盐二水
镍,[碳酸(2-)-κO]-
镁(1-甲基-3-氧代-丁-1-烯基)碳酸氢酯
锌氮烷碳酸盐
锆碳酸盐氧化物
锂(1-羧基环丙基)锂
铵铜碳酸盐
铯碳酸氢钠
铝镁加
铝镁加
铝碳酸镁
铝碳酸镁
钠脲氯酸盐
钠甲基碳酸酯
钙钠碳酸氢盐氟化物
钙四镁钠碳酸氢盐三碳酸盐四氢氧化物
钐(+3)阳离子碳酸酯
重质碳酸镁
重碳酸钠-13C
酸氧(-2)阴离子铅杂亚酸碳
酮羧酸
邻苯二甲酸氢壬酯
过氧碳酸钠
过氧碳酸二钠盐
过氧碳酸,O,O'-1,6-亚己基-OO,OO'-二叔丁基酯
过氧化脲素
过氧化二碳酸双十四酯
过氧化二碳酸双十六酯
过氧化二碳酸二硬脂酰酯
过氧化二碳酸二环己酯
过氧化二碳酸二正丁酯
过氧化二碳酸二异丙酯
过氧化二碳酸二仲丁酯
过氧化二碳酸二乙酯
过氧化二碳酸二-3-甲氧基丁酯
过氧化二碳酸二(2-乙基己)酯
过氧化(2-乙基己基)碳酸叔戊酯
过氧二碳酸二十三烷酯
过氧二碳酸二丙基酯
达比加群酯杂质41
达比加群酯杂质22
达比加群杂质36
达比加群杂质19
辛酰脲
辛基辛氧基甲基碳酸酯
辛基脲
轻质碳酸镁
起始原料2杂质B