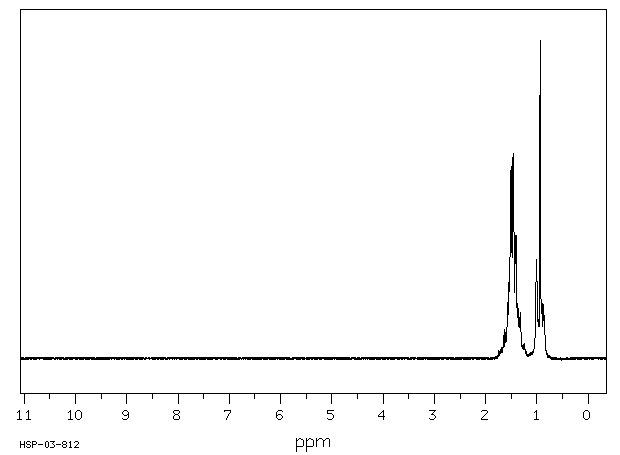
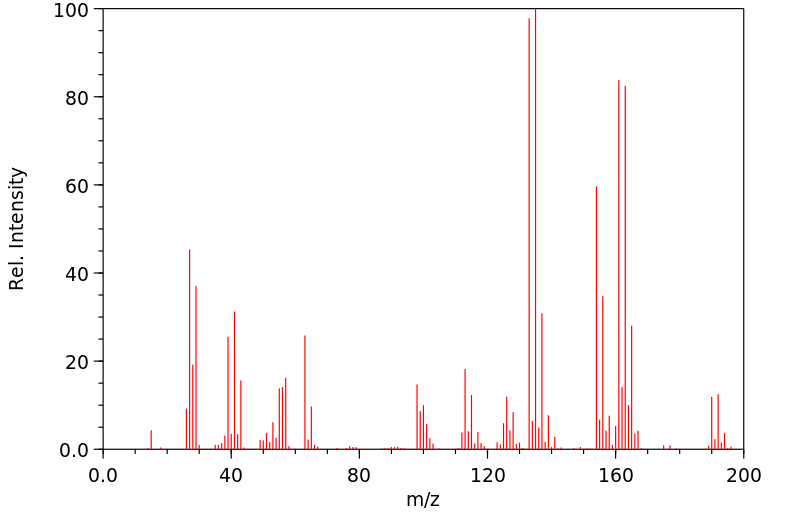
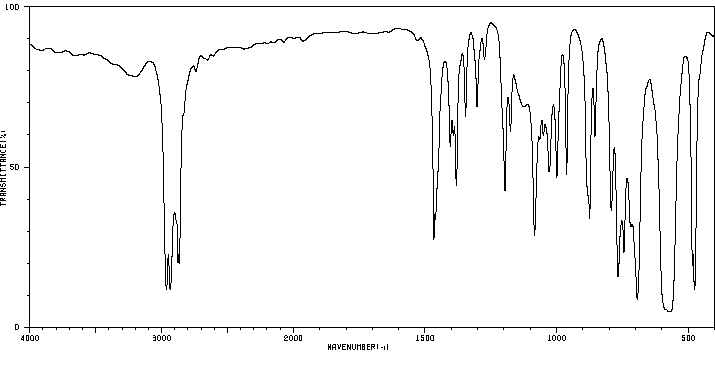
1.1 产品标识符 : ButyltrichlorOSi
LAne
化学品俗名或商品名
1.2 鉴别的其他方法 无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途 仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述 2.1 GHS分类 易燃液体 (类别3)
急性毒性, 经口 (类别4)
皮肤腐蚀 (类别1B)
严重的眼损伤 (类别1)
2.2 GHS 标记要素,包括预防性的陈述 危害类型象形图
信号词 危险
危险申明
H226 易燃液体和蒸气
H302 吞咽有害。
H314 造成严重皮肤灼伤和眼损伤。
警告申明
预防
P210 远离热源/火花/明火/热表面。- 禁止吸烟。
P233 保持容器密闭。
P240 容器和接收设备接地/等势连接。
P241 使用防爆的电气/ 通风/ 照明 设备。
P242 只能使用不产生火花的工具。
P243 采取防止静电放电的措施。
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮
水或吸烟。
P280 戴防护手套/ 穿防护服/ 戴防护眼罩/ 戴防护面具。
措施
P301 + P312 如果吞下去了: 如感觉不适,呼救解毒中心或看医生。
P301 + P330 + P331 如误吞咽:漱口。不要诱导呕吐。
P303 + P361 + P353 如皮肤(或头发)沾染:立即去除/ 脱掉所有沾染的衣服。用
水清洗皮肤/
淋浴。
P304 + P340 如果吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如进入眼睛:用
水小心清洗几分钟。如戴隐形眼镜并可方便地取出,取出
隐形眼镜。继续冲洗。
P310 立即呼救解毒中心或医生。
P321 具体治疗(见本标签上提供的急救指导)。
P363 沾染的衣服清洗后方可重新使用。
P370 + P378 火灾时: 用干的砂子,干的
化学品或耐醇性的泡沫来灭火。
储存
P403 + P235 存放在通风良好的地方。保持低温。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 遇
水剧烈反应。
模块 3. 成分/组成信息 3.1 物 质 :
C4H9Cl3Si 分子式
: 191.56 g/mol
分子量
成分 浓度
ButyltrichlorOSi
LAne
-
化学文摘编号(CAS No.)
7521-80-4 EC-编号 231-381-3
模块 4. 急救措施 4.1 必要的急救措施描述 一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
在皮肤接触的情况下
立即脱掉污染的衣服和鞋子。 用肥皂和大量的
水冲洗。 请教医生。
在眼睛接触的情况下
用大量
水彻底冲洗至少15分钟并请教医生。
如果误服
禁止催吐。 切勿给失去知觉者从嘴里喂食任何东西。 用
水漱口。 请教医生。
4.2 最重要的症状和影响,急性的和滞后的 该物质对粘膜组织和上呼吸道、眼睛和皮肤破坏巨大。, 痉挛,发炎,咽喉肿痛, 痉挛,发炎,支气管炎, 肺炎,
肺
水肿, 灼伤感:, 咳嗽, 喘息, 喉炎, 呼吸短促, 头痛, 恶心
4.3 及时的医疗处理和所需的特殊处理的说明和指示 无数据资料
模块 5. 消防措施 5.1 灭火介质 灭火方法及灭火剂
干粉
5.2 源于此物质或混合物的特别的危害 碳氧化物,
氯化氢气体,
二氧化硅 5.3 救火人员的预防 如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息 无数据资料
模块 6. 泄露应急处理 6.1 人员的预防,防护设备和紧急处理程序 使用个人防护设备。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 移去所有火源。
将人员撤离到安全区域。 防范蒸汽积累达到可爆炸的浓度,蒸汽能在低洼处积聚。
6.2 环境预防措施 在确保安全的条件下,采取措施防止进一步的泄漏或溢出。 不要让产物进入下
水道。
6.3 抑制和清除溢出物的方法和材料 用防电真空清洁器或湿的刷子将溢出物收集起来并放置到容器中去,根据当地规定处理(见第13部分)。
不要用
水冲洗。
6.4 参考其他部分 丢弃处理请参阅第13节。
模块 7. 操作处置与储存 7.1 安全操作的注意事项 避免接触皮肤和眼睛。 防止吸入蒸汽和烟雾。
切勿靠近火源。-严禁烟火。采取防静电生成的措施。
7.2 安全储存的条件,包括任何不兼容性 贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
贮存期间严禁与
水接触。
储存于氮气中 遇
水剧烈反应。
7.3 特定用途 无数据资料
模块 8. 接触控制/个体防护 8.1 控制参数 最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制 适当的技术控制
根据工业卫生和安全使用规则来操作。 休息以前和工作结束时洗手。
人身保护设备
眼/面保护
紧密装配的防护眼镜请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/E
EC规定和从它衍生出来的EN 376标准。
身体保护
全套防
化学试剂工作服, 阻燃防静电防护服,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性 9.1 基本的理化特性的信息 a) 外观与性状
形状: 液体
颜色: 浅红
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
149 °C - lit.
g) 闪点
45 °C - 闭杯
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
1.16 g/cm3 在 25 °C
n)
水溶性
无数据资料
o)
辛醇/
水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性 10.1 反应性 无数据资料
10.2 化学稳定性 无数据资料
10.3 危险反应的可能性 遇
水剧烈反应。
10.4 避免接触的条件 热,火焰和火花。 暴露在
潮湿中。
10.5 不兼容的材料 强氧化剂强氧化剂,
水 10.6 危险的分解产物 其它分解产物 - 无数据资料
模块 11. 毒理学资料 11.1 毒理学影响的信息 急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 该物质对组织、粘膜和上呼吸道破坏力强
摄入 误吞对人体有害。 引致灼伤。
皮肤 如果通过皮肤吸收可能是有害的。 引起皮肤烧伤。
眼睛 引起眼睛烧伤。
接触后的征兆和症状
该物质对粘膜组织和上呼吸道、眼睛和皮肤破坏巨大。, 痉挛,发炎,咽喉肿痛, 痉挛,发炎,支气管炎, 肺炎,
肺
水肿, 灼伤感:, 咳嗽, 喘息, 喉炎, 呼吸短促, 头痛, 恶心
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料 12.1 毒性 无数据资料
12.2 持久存留性和降解性 无数据资料
12.3 生物积累的潜在可能性 无数据资料
12.4 土壤中的迁移 无数据资料
12.5 PBT 和 vPvB的结果评价 无数据资料
12.6 其它不利的影响 无数据资料
模块 13. 废弃处置 13.1 废物处理方法 产品
在装备有加力燃烧室和洗刷设备的
化学焚烧炉内燃烧处理,特别在点燃的时候要注意,因为此物质是高度易燃
性物质 将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息 14.1 UN编号 欧洲陆运危规: 1747 国际海运危规: 1747 国际空运危规: 1747
14.2 联合国(UN)规定的名称 欧洲陆运危规: BUTYLTRICHLOROSI
LANE
国际海运危规: BUTYLTRICHLOROSI
LANE
国际空运危规: ButyltrichlorOSi
LAne
客运飞机: 不允许运输
14.3 运输危险类别 欧洲陆运危规: 8 (3) 国际海运危规: 8 (3) 国际空运危规: 8 (3)
14.4 包裹组 欧洲陆运危规: II 国际海运危规: II 国际空运危规: II
14.5 环境危险 欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防 无数据资料
模块 15 - 法规信息 N/A
模块16 - 其他信息 N/A