2,2,2-三氯-1,1-二甲基乙基二氯亚磷酸酯 | 39177-74-7
中文名称
2,2,2-三氯-1,1-二甲基乙基二氯亚磷酸酯
中文别名
2,2,2-三氯-1,1-二甲基乙基亚磷酰二氯
英文名称
2,2,2-trichloro-1,1-dimethylethyldichloro phosphite
英文别名
2,2,2-trichloro-1,1-dimethyl-ethyl phosphorodichlorodite;1,1-dimethyl-2,2,2-trichloroethyl phosphorodichloridite;2,2,2-trichloro-1,1-dimethylethyl phosphorodichloridite;1,1,1-trichloro-2-methyl-prop-2-yl dichlorophosphite;2,2,2-trichloro-1,1-dimethylethyl dichlorophosphite;2,2,2-trichloro-tert-butyl phosphodichloridite;dichloro-(1,1,1-trichloro-2-methylpropan-2-yl)oxyphosphane
CAS
39177-74-7
化学式
C4H6Cl5OP
mdl
——
分子量
278.33
InChiKey
XDLCMHDXDUGTMG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
密度:1.528 g/mL at 25 °C(lit.)
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:11
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:8
-
危险品运输编号:UN 1760
-
海关编码:2909199090
-
包装等级:III
-
危险类别:8
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Dialkyl hydrogen phosphites and dialkyl phosphorochloridites derived from chlorine-substituted alcohols摘要:DOI:10.1007/bf01151360
-
作为产物:描述:参考文献:名称:Kellner,H.A.; Ugi,I., Zeitschrift fur Naturforschung, Teil B: Anorganische Chemie, Organische Chemie, 1979, vol. 34, p. 1159 - 1161摘要:DOI:
文献信息
-
Synthesis of two structural analogues of the smallest antibiotically active degradation product of moenomycin a作者:Heidemarie Hohgardt、Wolfgang Dietrich、Harald Kühne、Dietrich Müller、Detlef Grzelak、Peter WelzelDOI:10.1016/s0040-4020(01)81436-8日期:1988.1The disacchande analogues 19 and 2 of moenomycin A have been synthesized. In contrast to the moenomycin A degradation product 1, synthetic conpounds 19 and 2 do not show antibiotic activity.已经合成了莫诺霉素A的令人不快的类似物19和2。与莫那霉素A降解产物1相反,合成化合物19和2没有显示抗生素活性。
-
Studies on the Synthesis of Di- and Trisaccharide Analogues of Moenomycin A. Modifications in Unit E and in the Lipid Part作者:Guangbin Yang、Madina Mansourova、Lothar Hennig、Matthias Findeisen、Ramona Oehme、Sabine Giesa、Peter WelzelDOI:10.1002/hlca.200490161日期:2004.7Routes allowing the synthesis of moenomycin analogues with one modified sugar component and with new lipid parts were developed (see 10c, 12c, 16b, and 20b in Schemes 2–4). It is anticipated that such analogues will be useful for studying the mode of action of the moenomycin-type transglycosylase inhibitors in detail and for preparing analogues with improved pharmacokinetic properties.路由允许moenomycin类似物的合成与一种经修饰的糖组分,并用新的脂质部分被开发(见图10C,12C,16B,和20B在方案2 - 4)。预期此类类似物可用于详细研究莫能霉素型转糖基化酶抑制剂的作用方式以及用于制备具有改善的药代动力学性质的类似物。
-
Some developments in the phosphitetriester method for synthesis of oligonucleotides作者:R.L. Letsinger、E.P. Groody、N. Lander、T. TanakaDOI:10.1016/0040-4020(84)85112-1日期:1984.1phosphite-triester synthesis of oligonucleotides. Use of this group enables one to simplify procedures for preparing active nucleoside phosphorochloridite reagents, to remove oligonucleotide phosphotriester intermediates intact from a solid support, and to conduct block syntheses in solution or on a silica support viaphosphite chemistry. Deprotection can be achieved by warming the trihaloethyl phosphotriesters with
-
Synthesis of a trisaccharide analogue of moenomycin A12 Implications of new moenomycin structure-activity relationships作者:Olaf Ritzeler、Lothar Hennig、Matthias Findeisen、Peter Welzel、Dietrich Müller、Astrid Markus、Guy Lemoine、Maxime Lampilas、Jean van HeijenoortDOI:10.1016/s0040-4020(96)01115-5日期:1997.2A trisaccharide analogue of moenomycin A, 9a, has been synthesized and has found to be antibiotically inactive. This compound differs from an active compound, 9b, solely by the exchange NHAc→OH in unit C. A binding model for moenomycin-type transglycosylase inhibitors at the enzyme penicillin binding protein is proposed.
-
Moenomycin A - Structure-activity relations synthesis of the D-galacturonamide analogue of the smallest antibiotically active degradation product of moenomycin A作者:Uwe Möller、Kurt Hobert、Astrid Donnerstrag、Petra Wagner、Dietrich Müller、Hans-Wolfram Fehlhaber、Astrid Markus、Peter WelzelDOI:10.1016/s0040-4020(01)80351-3日期:——Compound 10c which is the galacturonamide analogue of 2, the smallest degradation product of moenomycin A (1) with full antibiotic activity, has been synthesized. 10c is devoid of antibiotic activity.合成了化合物10c,它是2的半乳糖醛酰胺类似物,这是具有完全抗生素活性的莫诺霉素A(1)的最小降解产物。10c没有抗生素活性。
表征谱图
-
氢谱1HNMR
-
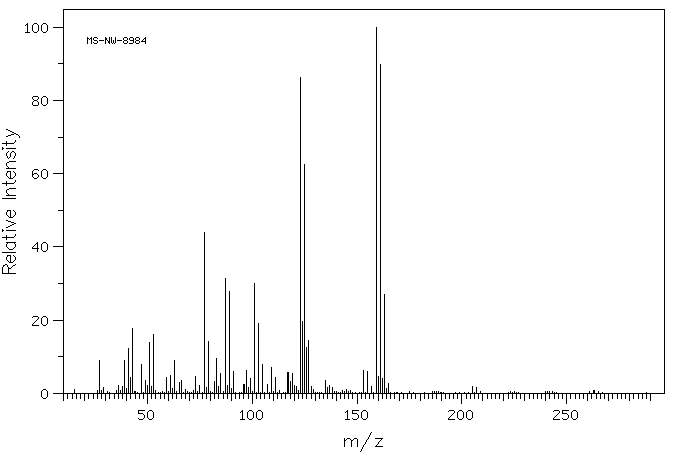
质谱MS
-
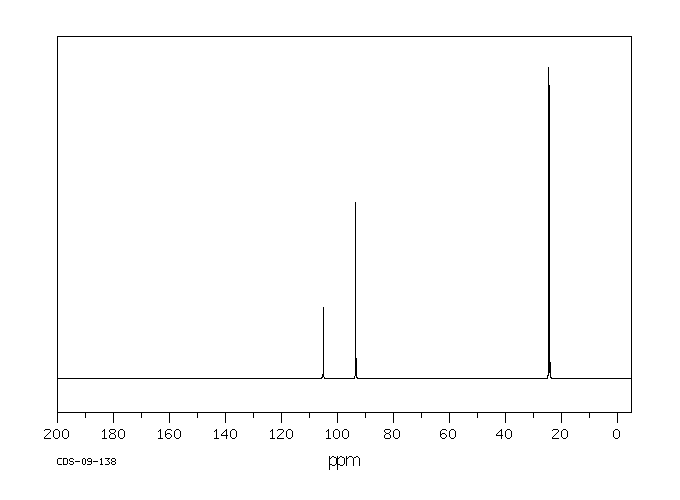
碳谱13CNMR
-
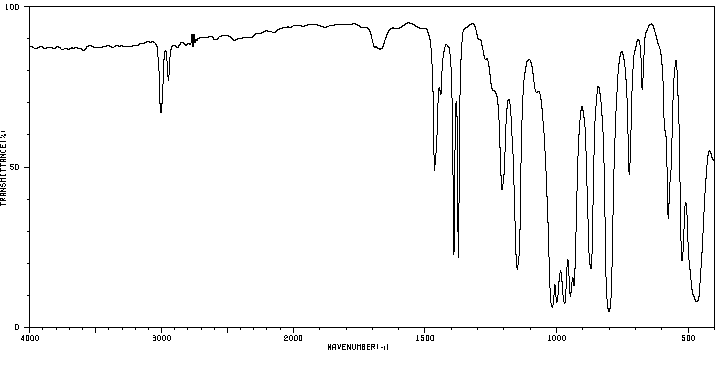
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式1,4-二氯-2-甲基-2-丁烯
顺式1,1,1,5-四氯-4-甲基-3-戊烯
顺式-7-甲基环庚-2-烯基氯
顺式-4-甲基环庚-2-烯基氯
顺式-1-氨基-4-氯-2-丁烯
顺式-1,4-二氯-2-丁烯
顺-6-氯-2-己烯
顺-4-氯-2-丁烯胺盐酸盐
锡烷,二(4-氯丁基)羰基-
锡烷,三氯(2-乙烯基壬基)-
重氮乙酰氯
辛基癸基二甲基氯化铵
聚乙烯胺
羟肟基乙酰氯
磷亚胺三氯化,[1,2,2,2-四氯-1-(三氯甲基)乙基]-
硫代氯甲酸-O-辛酯
癸醛,2,2-二氯-
甲醛与氨和氯乙烷的聚合物
甲基(2E)-2-(3-氯-2-丁烷亚基)肼羧酸酯
环己烷,(氯甲基)-
环丙烷,2-丁基-1-氯-1-(1-戊炔基)-,顺-
环丙烷,1,2-二溴-3,3-二氯-1,2-二丙基-,反-
环丙烷,1,1-二溴-2,3-二氯-2,3-二乙基-,反-
环丙烷,1,1-二氯-3-(氯甲基)-2,2-二甲基-
环丙烷,1,1,2,3-四氯-2,3-二甲基-,反-
环丙基甲基氯
环丁基氯
特比萘芬杂质17
溴代二氯丁烷
油酰氯
油酰氯
水合2-氯乙醛
氯螺戊烷
氯磺酸-(2,3-二氯丙酯)
氯甲醇
氯甲氧基
氯甲基自由基
氯甲基环丁烷
氯甲基氯磺酸酯
氯甲基二氯甲基醚
氯甲基(甲基)次磷酰氯
氯环辛烷
氯环癸烷
氯环庚烷
氯环丙烷
氯十七烷
氯化链烷烃
氯化环十二烷
氯化新戊烷
氯代环戊烷