2,5-二氯-4-甲氧基苯酚 | 18113-14-9
中文名称
2,5-二氯-4-甲氧基苯酚
中文别名
——
英文名称
2,5-dichloro-4-methoxyphenol
英文别名
2,5-Dichlor-4-methoxy-phenol
CAS
18113-14-9
化学式
C7H6Cl2O2
mdl
——
分子量
193.029
InChiKey
JXKFAPUUGWQXHX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:1399.9
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:29.5
-
氢给体数:1
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:描述:四甲基甲烷二胺 、 2,5-二氯-4-甲氧基苯酚 生成参考文献:名称:Dimethylaminomethylation of phenols and α-naphthols摘要:DOI:10.1007/bf00761182
-
作为产物:描述:参考文献:名称:Akerman et al., Journal of Applied Chemistry, 1953, vol. 3, p. 416摘要:DOI:
文献信息
-
Synthesis of o-chlorophenols via an unexpected nucleophilic chlorination of quinone monoketals mediated by N,N′-dimethylhydrazine dihydrochloride作者:Zhiwei Yin、Jinzhu Zhang、Jing Wu、Riana Green、Sihan Li、Shengping ZhengDOI:10.1039/c4ob00391h日期:——An unexpected nucleophilic chlorination of a quinone monoketal while carrying out a pyrazolidine synthesis has led to a general preparation of multisubstituted phenols. The products are obtained in good to high yields under mild conditions. The bridged pyrazolidines that were the original targets are obtained in the presence of a protic solvent.
-
Efficient Coupling Reaction of Quinone Monoacetal with Phenols Leading to Phenol Biaryls作者:Tohru Kamitanaka、Koji Morimoto、Kohei Tsuboshima、Daichi Koseki、Hitoho Takamuro、Toshifumi Dohi、Yasuyuki KitaDOI:10.1002/anie.201608013日期:2016.12.12phenol biaryls by the cross‐couplings of quinone monoacetals (QMAs) and phenols is reported. The Brønsted acid catalytic system in 1,1,1,3,3,3‐hexafluoro‐2‐propanol was found to be particularly efficient for this transformation. This reaction can be extended to the synthesis of various phenol biaryls, including sterically hindered biaryls, with yields ranging from 58 to 90 % under mild reaction conditions据报道,通过醌单缩醛(QMA)和酚类的交叉偶联可以简单而有效地合成苯酚联芳基。发现在1,1,1,3,3,3-六氟-2-丙醇中的布朗斯台德酸催化体系对于这种转化特别有效。该反应可以扩展到各种苯酚联芳基的合成,包括空间位阻联芳基,在温和的反应条件下,以高度区域特异性的方式,收率范围为58%至90%。
-
Antiviral activity of some .beta.-diketones. 1. Aryl alkyl diketones. In vitro activity against both RNA and DNA viruses作者:Guy D. Diana、U. Joseph Salvador、Ethel S. Zalay、Robert E. Johnson、Joseph C. Collins、David Johnson、William B. Hinshaw、Roman R. Lorenz、William H. Thielking、Francis PancicDOI:10.1021/jm00216a003日期:1977.6The discovery that 4-[3-ethyl-6-[(3,4-methylenedioxy)phenyl]-3-hexenyl]-3,5-heptanedione (40) exhibited an in vitro inhibitory effect against equine rhinovirus led to a structure--activity study to establish the criteria for optimum activity. Modification of the bridge included removal of the ethyl group and reduction of the double bond. The heptanedione was replaced with hexanedione and pentanedione with a minimal effect. The effect of replacing the heptanedione with beta-keto esters and monoketones was also investigated. Maintaining the hexamethylene bridge and heptanedione, the methylenedioxy group was replaced with various substitutents. In general, most substituents did not adversely affect activity particularly against equine rhinovirus although there was some variation in activity against herpesvirus. Strongly hydrophilic groups significantly reduced activity. Finally, the effect of varying the length of the alkyl bridge was examined in the 4-hydroxyphenyl series, where peak activity was attained with n = 8.
-
Reaction of Trialkyl Phosphites with p-Benzoquinone and with Other Symmetrically Substituted p-Quinones. A New Synthesis of Hydroquinone Monoalkyl Ethers<sup>1,2</sup>作者:Fausto Ramirez、Eugene H. Chen、Samuel DershowitzDOI:10.1021/ja01525a059日期:1959.8
-
US4137273A申请人:——公开号:US4137273A公开(公告)日:1979-01-30
表征谱图
-
氢谱1HNMR
-
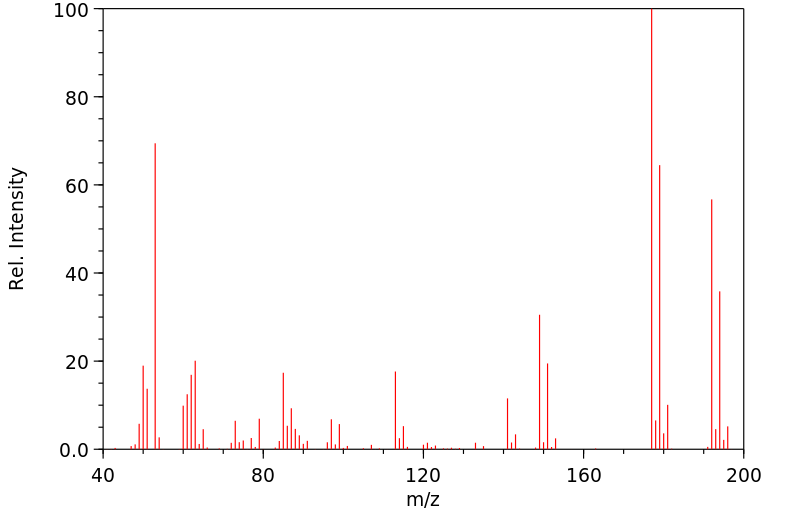
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚