2,5-双甲氧酰基-3,4-二苯基环戊二烯酮 | 16691-79-5
中文名称
2,5-双甲氧酰基-3,4-二苯基环戊二烯酮
中文别名
2,5-二(甲氧羰基)-3,4-二苯基环戊二酮;3,4-二苯基环戊二烯酮-2,5-二羧酸二甲酯;2,5-双(甲氧酰基)-3,4-二苯基环戊二烯酮
英文名称
2,5-bis(methoxycarbonyl)-3,4-diphenylcyclopentadienone
英文别名
2,5-dicarbomethoxy-3,4-diphenylcyclopentadienone;2-oxo-4,5-diphenyl-cyclopenta-3,5-diene-1,3-dicarboxylic acid dimethyl ester;dimethyl 2-oxo-4,5-diphenylcyclopenta-3,5-diene-1,3-dicarboxylate
CAS
16691-79-5
化学式
C21H16O5
mdl
MFCD00111095
分子量
348.355
InChiKey
NTXFYWYVVGMAGT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:175 °C
-
沸点:445.4±45.0 °C(Predicted)
-
密度:1.302±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:26
-
可旋转键数:6
-
环数:3.0
-
sp3杂化的碳原子比例:0.095
-
拓扑面积:69.7
-
氢给体数:0
-
氢受体数:5
安全信息
-
海关编码:2918300090
-
储存条件:室温且干燥环境中保存
SDS
2,5-Bis(methoxycarbonyl)-3,4-
diphenylcyclopentadienone
SAFETY DATA SHEET
Section 1. IDENTIFICATION OF THE SUBSTANCE OR MIXTURE AND OF THE SUPPLIER
Product name: 2,5-Bis(methoxycarbonyl)-3,4-diphenylcyclopentadienone
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
Not classified
PHYSICAL HAZARDS
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
Hazard statement None
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
2,5-Bis(methoxycarbonyl)-3,4-diphenylcyclopentadienone
Component(s):
Percent: ....
16691-79-5
CAS Number:
Synonyms: Dimethyl 3,4-Diphenylcyclopentadienone-2,5-dicarboxylate , 3,4-
Diphenylcyclopentadienone-2,5-dicarboxylic Acid Dimethyl Ester
Chemical Formula: C21H16O5
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Get medical advice/attention if you feel unwell. Rinse mouth.
Ingestion:
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
2,5-Bis(methoxycarbonyl)-3,4-
diphenylcyclopentadienone
Section 5. FIRE-FIGHTING MEASURES
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: crystal - powder
Color: Yellow - Yellow red
Odor: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Stability:
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
2,5-Bis(methoxycarbonyl)-3,4-
diphenylcyclopentadienone
Section 10. STABILITY AND REACTIVITY
Hazardous Decomposition Carbon monoxide, Carbon dioxide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not Listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
2,5-Bis(methoxycarbonyl)-3,4-
diphenylcyclopentadienone
SECTION 16 - ADDITIONAL INFORMATION
N/A
diphenylcyclopentadienone
SAFETY DATA SHEET
Section 1. IDENTIFICATION OF THE SUBSTANCE OR MIXTURE AND OF THE SUPPLIER
Product name: 2,5-Bis(methoxycarbonyl)-3,4-diphenylcyclopentadienone
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
Not classified
PHYSICAL HAZARDS
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
None
Pictograms or hazard symbols
Signal word No signal word
Hazard statement None
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
2,5-Bis(methoxycarbonyl)-3,4-diphenylcyclopentadienone
Component(s):
Percent: ....
16691-79-5
CAS Number:
Synonyms: Dimethyl 3,4-Diphenylcyclopentadienone-2,5-dicarboxylate , 3,4-
Diphenylcyclopentadienone-2,5-dicarboxylic Acid Dimethyl Ester
Chemical Formula: C21H16O5
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Get medical advice/attention if you feel unwell. Rinse mouth.
Ingestion:
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
2,5-Bis(methoxycarbonyl)-3,4-
diphenylcyclopentadienone
Section 5. FIRE-FIGHTING MEASURES
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: crystal - powder
Color: Yellow - Yellow red
Odor: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Stability:
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
2,5-Bis(methoxycarbonyl)-3,4-
diphenylcyclopentadienone
Section 10. STABILITY AND REACTIVITY
Hazardous Decomposition Carbon monoxide, Carbon dioxide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not Listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
2,5-Bis(methoxycarbonyl)-3,4-
diphenylcyclopentadienone
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2,5-dicarbomethoxy-4-hydroxyl-3,4-diphenylcyclopent-2-en-1-one 16691-78-4 C21H18O6 366.37 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— dimethyl 4,5-diphenylcyclopenta-3,5-diene-1,3-dicarboxylate 1312150-19-8 C21H18O4 334.372 —— 2,5-dicarbomethoxy-4-hydroxyl-3,4-diphenylcyclopent-2-en-1-one 16691-78-4 C21H18O6 366.37 —— Dimethyl 1-anilino-2-oxo-4,5-diphenylcyclopent-3-ene-1,3-dicarboxylate —— C27H23NO5 441.483 —— Dimethylester 51544-86-6 C27H23NO5 441.483
反应信息
-
作为反应物:描述:2,5-双甲氧酰基-3,4-二苯基环戊二烯酮 在 sodium tetrahydroborate 、 水 作用下, 以 乙醇 为溶剂, 反应 0.42h, 以57%的产率得到dimethyl 4,5-diphenylcyclopenta-3,5-diene-1,3-dicarboxylate参考文献:名称:Demonstration of the facile reversibility of fulvene formation摘要:Cleavage of fulvenes under mild conditions and interchange between electron-deficient fulvenes and their constituent cyclopentadienes and imines is demonstrated for the first time. A series of cyclopentadienes and imines are investigated to probe the dependence of fulvene equilibration on structure. The exchange of one fulvene for another is demonstrated in the first reported example of transfulvenation. Finally, the metathesis of two fulvenes to generate all four possible cross products is shown. (C) 2011 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2011.03.094
-
作为产物:参考文献:名称:Eistert,B.; Thommen,A.J., Chemische Berichte, 1971, vol. 104, p. 3048 - 3061摘要:DOI:
-
作为试剂:描述:2-phenoxy-2-ethyl-2,3-dihydro-1,3,2λ5-benzoxazaphosphole 在 2,5-双甲氧酰基-3,4-二苯基环戊二烯酮 作用下, 以 苯 为溶剂, 反应 0.02h, 以92%的产率得到Dimethyl 2-oxo-4,5-diphenylcyclopent-3-ene-1,3-dicarboxylate参考文献:名称:Fuzhenkova, A. V.; Tyryshkin, N. I.; Terent'eva, S. A., Russian Journal of General Chemistry, 1993, vol. 63, # 5.1, p. 740 - 742摘要:DOI:
文献信息
-
Frontier-controlled cycloaddition reactions of cyclopentadienones having electron-donating or -attracting substituents: configuration of adducts and kinetic studies作者:Masato Mori、Ayako Hayamizu、Ken KanematsuDOI:10.1039/p19810001259日期:——inverse electron demand. By contrast, in the correlation for the cycloaddition of (1b) with (2a–h), two lines with markedly different slopes were observed, which can be ascribed to a Diels–Alder reaction with neutral electron demand. The initial cycloadditions of (1a) and (1b) with tropone (29) gave the [4 + 6]π adducts (32a,b). Subsequently the adduct (32b) underwent oxy-Cope rearrangement to (35). The2,5-二甲氧基羰基-和2,5-二乙基-3,4-二苯基环戊二烯酮[CPC(1a)和EPC(1b)]与对位取代的苯乙烯(2a–h)的环加成反应产生的主要加合物的立体化学证实降冰片二烯(7),降冰片烯(8),1,4-二氢-1,4-环氧萘(9),马来酸酐(10)和N-苯基马来酰亚胺(11)具有内构型。为了定义取代基对Diels-Alder反应速率的影响,对(1a,b)与一系列苯乙烯(2a-h)的反应性进行了动力学研究。(1a)与(2a–h)的环加成的对数k / k H值与冈本布朗的σp +相关常数和ρ值为–0.941。该结果表明,环加成反应被归类为具有反电子需求的Diels-Alder反应。相比之下,在(1b)与(2a–h)的环加成反应的相关性中,观察到两条斜率明显不同的线,这可以归因于具有中性电子需求的Diels–Alder反应。(1a)和(1b)与对苯二酚(29)的初始环加成反应产生[4 +
-
Effect of >CO···H–Ar interaction on endo/exo selectivity in the Diels–Alder reaction of phenyl-substituted cyclopentadienones作者:Yasuyuki Yoshitake、Hidetoshi Nakagawa、Masashi Eto、Kazunobu HaranoDOI:10.1016/s0040-4039(00)00602-x日期:2000.6e with S-allyl S-methyl dithiocarbonate gave the endo cycloadduct whereas phencyclone gave a mixture of the endo and exo cycloadducts. The X-ray analysis of the cycloadduct of phencyclone and S-allyl S-methyl dithiocarbonate indicated the presence of a short contact of Ar–H···OC< type. A possible role of the interaction in determining the endo/exo selectivity was discussed based on the X-ray crystallographic
-
Synthesis of a C<sub>56</sub>H<sub>40</sub> Hydrocarbon Bearing a 54-Carbon Framework of C<sub>60</sub>作者:Kung K. Wang、Yu-Hsuan Wang、Hua Yang、Novruz G. Akhmedov、Jeffrey L. PetersenDOI:10.1021/ol900749a日期:2009.6.18A C56H40 hydrocarbon possessing a 54-carbon framework represented on the surface of C60 was synthesized by solution-phase chemistry. The structure of this hydrocarbon has a 30-carbon core, which can be regarded as a partially hydrogenated [5,5]circulene, a C30H12 semibuckminsterfullerene, with only one of the carbon−carbon bonds remaining unconnected. The central 30-carbon core is fused with two indeno
-
Cascade Radical Cyclizations of Benzannulated Enyne−Allenes. Unusual Cleavage of a Benzene Ring Leading to Twisted 1,1‘-Dialkyl-9,9‘-bifluorenylidenes and Spiro[1<i>H</i>-cyclobut[<i>a</i>]indene-1,9‘-[9<i>H</i>]fluorenes]作者:Yonghong Yang、Jeffrey L. Petersen、Kung K. WangDOI:10.1021/jo030147j日期:2003.7.19'-[9H]fluorene] 20 apparently was produced via two intramolecular [2 + 2] cycloaddition reactions of the benzannulated enyne-allene moieties, generated in situ from the benzannulated enediynyl propargylic alcohols. The twisted 1,1'-dimethyl-9,9'-bifluorenylidene 33 and the spiro[1H-cyclobut[a]indene-1,9'-[9H]fluorene] 39 (trans/cis = 3:1) were likewise produced from 32 and 38, respectively.用亚硫酰氯处理苯并环化的二炔基炔丙醇16(异构体比例= 2:1),引发一系列反应,导致扭曲的1,1'-二丙基-9,9'-联芴基17,多环化合物18和19,和螺[1H-环丁[a]茚-1,9'-[9H]芴] 20(反式/顺式= 5:1)。从16到出乎意料的17的转变大概涉及苯甲环烯-烯丙烯21的初始形成,然后进行C(2)-C(6)环化反应和分子内自由基-自由基偶联反应,从而形成正式的Diels- Alder加合物23。重复该序列,然后提供24。连接具有丙基取代基的两个碳的键的断裂得到25。随后,连接两个中心五元环的碳-碳键的旋转随后得到反式异构体26 。26可能被氧气氧化,然后水解,然后生成17。有趣的是,导致17的途径涉及苯环的不寻常裂解。17的X射线晶体结构表明,其对于连接两个联芴基片段的碳-碳双键具有45.2度的扭转角。螺[1H-环丁[a]茚-1,9'-[9H]芴] 20显然是由苯并环烯炔-丙二烯部分的两个分子内[2
-
Studies on Seven-Membered Heterocycles. XXXIV. Syntheses and Reactions of 1-Benzosilepines, 1-Benzogermepines, 1-Benzophosphepines, and 1-Benzarsepines.作者:Shin-ichi SHIRATORI、Shuji YASUIKE、Jyoji KURITA、Takashi TSUCHIYADOI:10.1248/cpb.42.2441日期:——Flash vacuum pyrolysis of 2a, 7b-dihydrocyclobuta[b]-1-benzometalloles (6a-e), prepared from the corresponding 1-benzometalloles (3) containing Si, Ge, P, or As via three steps, resulted in valence isomerization with ring-opening to give 1, 1-dimethyl-1-benzosilepine (7a), 1-methyl-1-phenyl-1-benzosilepine (7b), 1, 1-dimethyl-1-benzogermepine (7c), 1-phenyl-1-benzophosphepine 1-oxide (7d), and 1-phenyl-1-benzarsepine 1-oxide (7e). The oxides (7d, e), on treatment with trichlorosilane, underwent deoxygenation to afford 1-phenyl-1-benzophosphepine (7f) and 1-phenyl-1-benzarsepine (7g). The 1-benzometallepines (7a-g) thus obtained are hitherto unknown heterocyclic ring compounds, and their thermal stabilities and several reactions were examined.采用快速真空热解法处理由硅、锗、磷或砷的1-苯并金属烯(3)经三步反应制备的2a, 7b-二氢环丁并[b]-1-苯并金属烯(6a-e),导致价态异构化及环的开裂,得到1, 1-二甲基-1-苯并硅宾烯(7a)、1-甲基-1-苯基-1-苯并硅宾烯(7b)、1, 1-二甲基-1-苯并锗宾烯(7c)、1-苯基-1-苯并膦宾烯1-氧化物(7d)和1-苯基-1-苯并砷宾烯1-氧化物(7e)。这些氧化物(7d, e)在三氯硅烷处理后,经历脱氧过程,产生1-苯基-1-苯并膦宾烯(7f)和1-苯基-1-苯并砷宾烯(7g)。获得的1-苯并金属烯(7a-g)是迄今为止未知的杂环化合物,且研究了它们的热稳定性和若干反应。
表征谱图
-
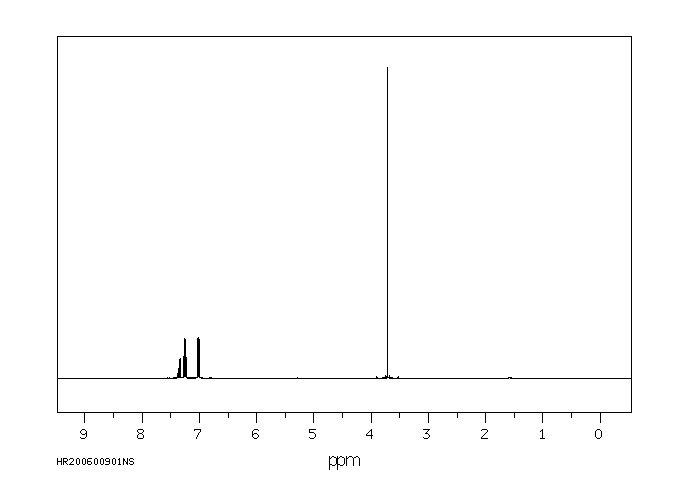
氢谱1HNMR
-
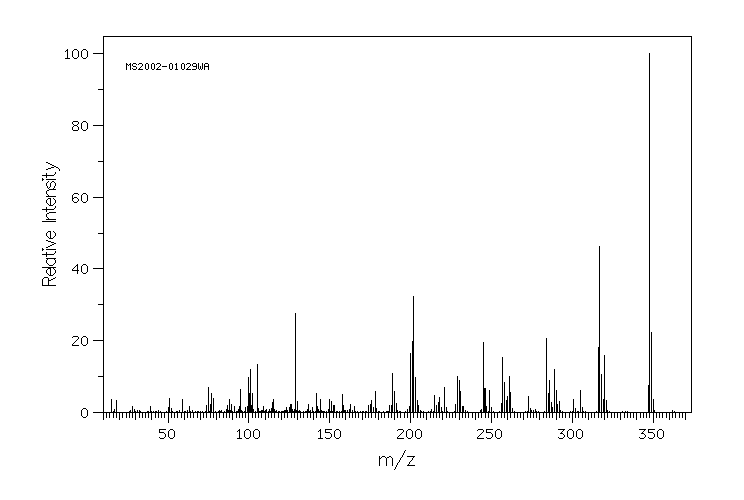
质谱MS
-
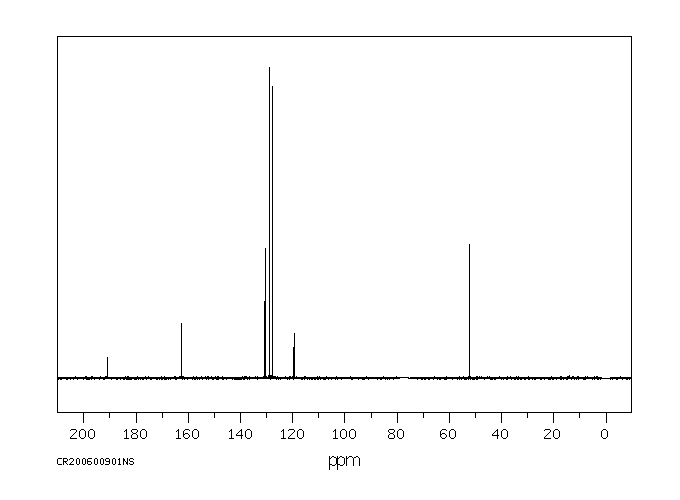
碳谱13CNMR
-
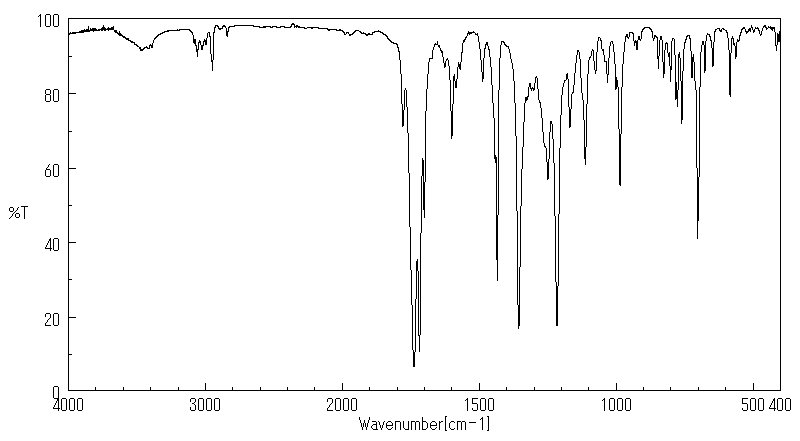
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E,Z)-他莫昔芬N-β-D-葡糖醛酸
(E/Z)-他莫昔芬-d5
(4S,5R)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S,5R,5''R)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(4R,5S)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4R,4''R,5S,5''S)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(1R,2R)-2-(二苯基膦基)-1,2-二苯基乙胺
鼓槌石斛素
黄子囊素
高黄绿酸
顺式白藜芦醇三甲醚
顺式白藜芦醇
顺式己烯雌酚
顺式-白藜芦醇3-O-beta-D-葡糖苷酸
顺式-桑皮苷A
顺式-曲札芪苷
顺式-二苯乙烯
顺式-beta-羟基他莫昔芬
顺式-a-羟基他莫昔芬
顺式-3,4',5-三甲氧基-3'-羟基二苯乙烯
顺式-1-(3-甲基-2-萘基)-2-(2-萘基)乙烯
顺式-1,2-双(三甲基硅氧基)-1,2-双(4-溴苯基)环丙烷
顺式-1,2-二苯基环丁烷
顺-均二苯乙烯硼酸二乙醇胺酯
顺-4-硝基二苯乙烯
顺-1-异丙基-2,3-二苯基氮丙啶
非洲李(PRUNUSAFRICANA)树皮提取物
阿非昔芬
阿里可拉唑
阿那曲唑二聚体
阿托伐他汀环氧四氢呋喃
阿托伐他汀环氧乙烷杂质
阿托伐他汀环(氟苯基)钠盐杂质
阿托伐他汀环(氟苯基)烯丙基酯
阿托伐他汀杂质D
阿托伐他汀杂质94
阿托伐他汀杂质7
阿托伐他汀杂质5
阿托伐他汀内酰胺钠盐杂质
阿托伐他汀中间体M4
阿奈库碘铵
锌(II)(苯甲醛)(四苯基卟啉)
银松素
铜酸盐(5-),[m-[2-[2-[1-[4-[2-[4-[[4-[[4-[2-[4-[4-[2-[2-(羧基-kO)苯基]二氮烯基-kN1]-4,5-二氢-3-甲基-5-(羰基-kO)-1H-吡唑-1-基]-2-硫代苯基]乙烯基]-3-硫代苯基]氨基]-6-(苯基氨基)-1,3,5-三嗪-2-基]氨基]-2-硫代苯基]乙烯基]-3-硫代
铒(III) 离子载体 I
铀,二(二苯基甲酮)四碘-
钾钠2,2'-[(E)-1,2-乙烯二基]二[5-({4-苯胺基-6-[(2-羟基乙基)氨基]-1,3,5-三嗪-2-基}氨基)苯磺酸酯](1:1:1)
钠{4-[氧代(苯基)乙酰基]苯基}甲烷磺酸酯
钠;[2-甲氧基-5-[2-(3,4,5-三甲氧基苯基)乙基]苯基]硫酸盐
钠4-氨基二苯乙烯-2-磺酸酯