2-chloro-3-(α-cyano-α-carbethoxy)methyl-1,4-napthoquinone | 25932-81-4
中文名称
——
中文别名
——
英文名称
2-chloro-3-(α-cyano-α-carbethoxy)methyl-1,4-napthoquinone
英文别名
2-chloro-3-(α-cyano-α-ethoxycarbonyl-methyl)-1,4-naphthoquinone;Ethyl-α-(2-chlor-1.4-dihydro-1.4-dioxo-3-naphthyl)-α-cyanoacetate;(3-chloro-1,4-dioxo-1,4-dihydro-naphthalen-2-yl)-cyano-acetic acid ethyl ester;3-Chlor-naphthochinon-(1.4)-cyanessigsaeure-(2)-aethylester;(3-Chlor-1,4-dioxo-1,4-dihydro-[2]naphthyl)-cyan-essigsaeure-aethylester;3-Chlor-2-(carbaethoxy-cyan-methyl)-naphthochinon-(1.4);Ethyl (3-chloro-1,4-dioxo-1,4-dihydronaphthalen-2-yl)(cyano)acetate;ethyl 2-(3-chloro-1,4-dioxonaphthalen-2-yl)-2-cyanoacetate
CAS
25932-81-4
化学式
C15H10ClNO4
mdl
——
分子量
303.702
InChiKey
MQFPUDFVKBOKAR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:121 °C(Solv: methanol (67-56-1))
-
沸点:462.4±45.0 °C(Predicted)
-
密度:1.41±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:21
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:84.2
-
氢给体数:0
-
氢受体数:5
反应信息
-
作为反应物:参考文献:名称:Reactions of Naphthoquinones with Malonic Ester and its Analogs. III. 1-Substituted Phthaloyl- and Phthaloylbenzopyrrocolines1摘要:DOI:10.1021/ja01562a048
-
作为产物:描述:2,3-二氯-1,4-萘醌 、 氰乙酸乙酯 在 ammonium hydroxide 作用下, 以 乙醇 为溶剂, 反应 0.33h, 以64%的产率得到2-chloro-3-(α-cyano-α-carbethoxy)methyl-1,4-napthoquinone参考文献:名称:Synthesis and cytotoxicity evaluation of 2-amino- and 2-hydroxy-3-ethoxycarbonyl- N -substituted-benzo[ f ]indole-4,9-dione derivatives摘要:Reaction between 2,3-dichloronaphthoquinone (I) and ethyl cyanoacetate or diethyl malonate under different conditions gave the starting materials, 2-chloro-3-(alpha-cyano-alpha-ethoxycarbonyl-methyl)-1,4-naphthoquinone (A) or 2-chloro-3-(diethoxycarbonyl-methyl)-1,4-naphthoquinone (B). The 2-amino-3-ethoxycarbonyl-N-substituted-benzo[f]indole-4,9-dione derivatives [A-(1-10)] and 2-hydroxy-3-ethoxycarbonyl-N-substituted-benzo[f]indole-4,9-dione derivatives [B-(1-12)] were prepared from compounds A and B, respectively, by using various alkyl-, and arylamines. The cytotoxic activities of the prepared compounds were evaluated by SRB (Sulforhodamine B) assay against the following tumor cell lines: A459 (human lung), SK-OV-3 (human ovarian), SK-MEL-2 (human melanoma), XF498 (human CNS), and HCT 15 (human colon). Many of the derivatives mentioned exhibited more potent cytotoxic effects against SK-OV-3 and XF498 than etoposide. Significantly, 2-amino-3-ethoxycarbonyl-N-(3-methyl-phenyl)-benzo[f]indole-4,9-dione (A-8) showed potent activity against all tumor cell lines, and in particular, its cytotoxic effect against SK-OV-3 was much higher than doxorubicin. (C) 2003 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0968-0896(03)00062-2
文献信息
-
Synthesis and application of ethyl 2-arylazo-4,9-dioxonaphtho[2,3-<i>b</i>]thiophene-3-carboxylate作者:D. W. Rangnekar、V. R. Kanetkar、G. S. Shankarling、J. V. MalankerDOI:10.1002/jhet.5570360103日期:1999.1Synthesis of ethyl 2-arylazo-4,9-dioxonaphtho[2,3-b]thiophene-3-carboxylate was achieved by diazotization of ethyl 2-amino-4,9-dioxonaphtho[2,3-b]thiophene-3-carboxylate and coupling with selected N,N-dialkylanilines. The key intermediate ethyl 2-amino-4,9-dioxonaphtho[2,3-b]thiophene-3-carboxylate was synthesized by the condensation of sodium salt of ethyl cyanoacetate with 2,3-dichloro-1,4-naphthoquinone
-
Liebermann, Chemische Berichte, 1899, vol. 32, p. 920作者:LiebermannDOI:——日期:——
-
Synthesis and Biological Evaluation of Benzo[f]indole-4,9-diones N-Linked to Carbohydrate Chains as New Type of Antitumor Agents作者:Flaviana Dias、Fabiana Guerra、Fernanda Lima、Yasmin de Castro、Vitor Ferreira、Vinícius Campos、Patrícia Fernandes、Anna CunhaDOI:10.21577/0103-5053.20200202日期:——
-
AKIBA M.; IKUTA S.; TAKADA T., HETEROCYCLES,1978, 9, NO 7, 813-818作者:AKIBA M.、 IKUTA S.、 TAKADA T.DOI:——日期:——
-
Akiba,M. et al., Heterocycles, 1978, vol. 9, # 7, p. 813 - 818作者:Akiba,M. et al.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
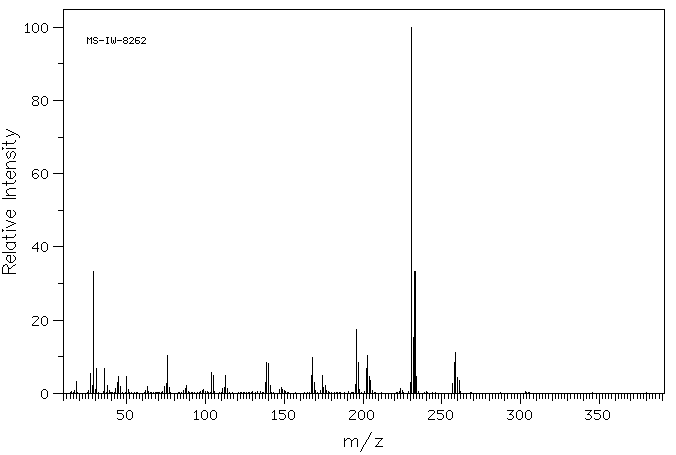
质谱MS
-
碳谱13CNMR
-
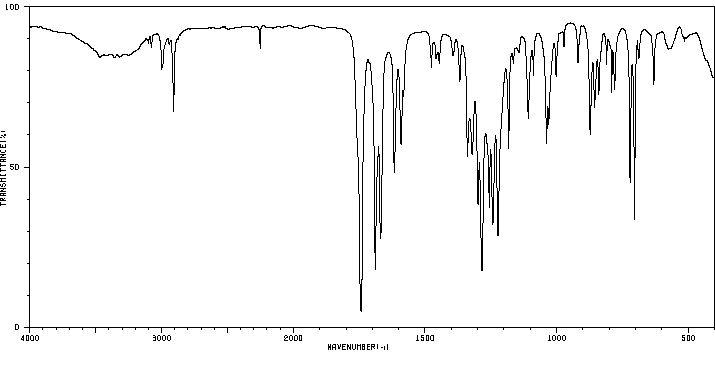
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮