1-benzyl-2,4,5-triphenyl-4,5-dihydro-1H-imidazole | 33722-47-3
中文名称
——
中文别名
——
英文名称
1-benzyl-2,4,5-triphenyl-4,5-dihydro-1H-imidazole
英文别名
1-Benzyl-2,4,5-triphenyl-4,5-dihydro-1H-imidazol;1-Benzyl-2,4,5-triphenyl-2-imidazoline;1-benzyl-2,4,5-triphenyl-4,5-dihydroimidazole
CAS
33722-47-3
化学式
C28H24N2
mdl
——
分子量
388.512
InChiKey
VSOVRPJUEZLLSA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:549.3±60.0 °C(Predicted)
-
密度:1.09±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):5.8
-
重原子数:30
-
可旋转键数:5
-
环数:5.0
-
sp3杂化的碳原子比例:0.11
-
拓扑面积:15.6
-
氢给体数:0
-
氢受体数:1
反应信息
-
作为产物:描述:苄胺 在 Mg-Al mixed oxide 、 air 作用下, 反应 36.0h, 生成 2,4,5-三苯基咪唑 、 1-benzyl-2,4,5-triphenyl-4,5-dihydro-1H-imidazole参考文献:名称:镁铝水滑石及其衍生的混合氧化物通过氧化-脱氢机理对亚胺化反应的催化活性摘要:以苯胺为模型反应,研究了Mg-Al水滑石和衍生的Mg-Al混合氧化物对苄基底物(苄胺和苄醇)的催化活性,从而建立了绿色且经济高效的催化(贵金属不含酒精的胺化反应和串联反应过程。Mg-Al混合氧化物(Mg / Al比= 3.0)被发现是用于亚胺化反应的有效催化剂,用于以高选择性合成交叉亚胺以及用于醇的串联反应。催化剂的碱性位点通过其官能团的氢(–NH 2)吸附苄基底物/ -OH)并激活(弱化)苄基CH键以消除氢化物。因此,碱性位点通过活化苄基底物以进行氧化脱氢(即,去质子化,然后借助氧气消除氢化物)来催化反应,以产生反应性的亚胺/羰基中间体,其随后与胺进行亲核反应。DOI:10.1039/c9nj06096k
文献信息
-
Synthesis of tetra-substituted imidazoles and 2-imidazolines by Ni(0)-catalyzed dehydrogenation of benzylic-type imines作者:Adrian Tlahuext-Aca、Oscar Hernández-Fajardo、Alma Arévalo、Juventino J. GarcíaDOI:10.1039/c4dt02313g日期:——performed to yield asymmetrical tetra-substituted imidazoles and 2-imidazolines. This was achieved with a single operational step while maintaining good selectivity and atom economy. The catalytic system shows low to moderate tolerance for fluoro-, trifluoromethyl-, methyl-, and methoxy-substituted benzylic-type imines. In addition, the substitution pattern at the N-heterocyclic products was easily
-
Photocatalytic coupling of amines to imidazoles using a Mo–ZnIn<sub>2</sub>S<sub>4</sub> catalyst作者:Min Wang、Lihua Li、Jianmin Lu、Nengchao Luo、Xiaochen Zhang、Feng WangDOI:10.1039/c7gc01728f日期:——we report a new route for the synthesis of substituted imidazoles via photocyclization of readily available amines at room temperature. The reaction is achieved by the visible-light-induced C–C/C–N bond coupling and subsequent dehydrogenation reaction over Mo–ZnIn2S4 as a heterogeneous photocatalyst. A wide range of amines were converted into the corresponding tri- and tetra-substituted imidazoles with
表征谱图
-
氢谱1HNMR
-
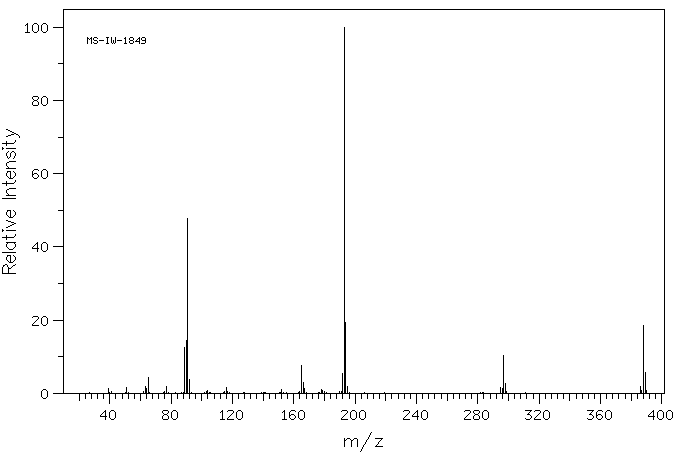
质谱MS
-
碳谱13CNMR
-
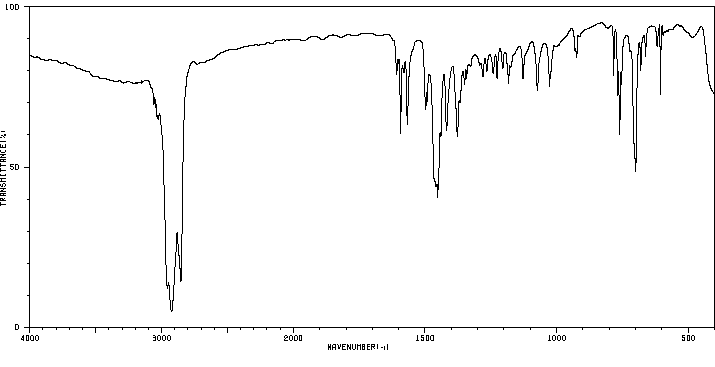
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E,Z)-他莫昔芬N-β-D-葡糖醛酸
(E/Z)-他莫昔芬-d5
(4S,5R)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S,5R,5''R)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(4R,5S)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4R,4''R,5S,5''S)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(1R,2R)-2-(二苯基膦基)-1,2-二苯基乙胺
鼓槌石斛素
黄子囊素
高黄绿酸
顺式白藜芦醇三甲醚
顺式白藜芦醇
顺式己烯雌酚
顺式-白藜芦醇3-O-beta-D-葡糖苷酸
顺式-桑皮苷A
顺式-曲札芪苷
顺式-二苯乙烯
顺式-beta-羟基他莫昔芬
顺式-a-羟基他莫昔芬
顺式-3,4',5-三甲氧基-3'-羟基二苯乙烯
顺式-1-(3-甲基-2-萘基)-2-(2-萘基)乙烯
顺式-1,2-双(三甲基硅氧基)-1,2-双(4-溴苯基)环丙烷
顺式-1,2-二苯基环丁烷
顺-均二苯乙烯硼酸二乙醇胺酯
顺-4-硝基二苯乙烯
顺-1-异丙基-2,3-二苯基氮丙啶
非洲李(PRUNUSAFRICANA)树皮提取物
阿非昔芬
阿里可拉唑
阿那曲唑二聚体
阿托伐他汀环氧四氢呋喃
阿托伐他汀环氧乙烷杂质
阿托伐他汀环(氟苯基)钠盐杂质
阿托伐他汀环(氟苯基)烯丙基酯
阿托伐他汀杂质D
阿托伐他汀杂质94
阿托伐他汀杂质7
阿托伐他汀杂质5
阿托伐他汀内酰胺钠盐杂质
阿托伐他汀中间体M4
阿奈库碘铵
锌(II)(苯甲醛)(四苯基卟啉)
银松素
铜酸盐(5-),[m-[2-[2-[1-[4-[2-[4-[[4-[[4-[2-[4-[4-[2-[2-(羧基-kO)苯基]二氮烯基-kN1]-4,5-二氢-3-甲基-5-(羰基-kO)-1H-吡唑-1-基]-2-硫代苯基]乙烯基]-3-硫代苯基]氨基]-6-(苯基氨基)-1,3,5-三嗪-2-基]氨基]-2-硫代苯基]乙烯基]-3-硫代
铒(III) 离子载体 I
铀,二(二苯基甲酮)四碘-
钾钠2,2'-[(E)-1,2-乙烯二基]二[5-({4-苯胺基-6-[(2-羟基乙基)氨基]-1,3,5-三嗪-2-基}氨基)苯磺酸酯](1:1:1)
钠{4-[氧代(苯基)乙酰基]苯基}甲烷磺酸酯
钠;[2-甲氧基-5-[2-(3,4,5-三甲氧基苯基)乙基]苯基]硫酸盐
钠4-氨基二苯乙烯-2-磺酸酯