2-亚硝基-1-萘酚-4-磺酸水合物 | 3682-32-4
中文名称
2-亚硝基-1-萘酚-4-磺酸水合物
中文别名
2-亚硝基-1-酚-4-磺酸;4-羟基-3-亚硝基-1-萘磺酸水合物
英文名称
2-nitroso-1-naphthol-4-sulphonic acid
英文别名
2-nitroso-1-naphthol-4-sulfonic acid;4-hydroxy-3-nitroso-naphthalene-1-sulfonic acid;4-Hydroxy-3-nitroso-naphthalin-1-sulfonsaeure;2-nitroso-1-hydroxy-naphthalene-4-sulphonic acid;1-Hydroxy-2-nitroso-naphthalin-4-sulfonsaeure;1-Hydroxy-2-nitrosonaphthalin-4-sulfonsaeure;1-Naphthalenesulfonic acid, 4-hydroxy-3-nitroso-;4-hydroxy-3-nitrosonaphthalene-1-sulfonic acid
CAS
3682-32-4
化学式
C10H7NO5S
mdl
MFCD00003933
分子量
253.235
InChiKey
GASPSNIEUBWWJU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:140-145 °C (dec.)
-
密度:1.65±0.1 g/cm3(Predicted)
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:17
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:112
-
氢给体数:2
-
氢受体数:6
安全信息
-
危险品标志:Xn
-
危险类别码:R20/21/22,R33
-
WGK Germany:3
-
海关编码:2908999090
-
安全说明:S36/37
SDS
4-羟基-3-亚硝基-1-萘磺酸水合物 修改号码:6
模块 1. 化学品
产品名称: 4-Hydroxy-3-nitroso-1-naphthalenesulfonic Acid Hydrate
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第4级
急性毒性(经皮) 第4级
急性毒性(吸入) 第4级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 吸入或皮肤接触或吞咽有害。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
4-羟基-3-亚硝基-1-萘磺酸水合物 修改号码:6
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。若感不适,呼叫解毒
中心/医生。
食入:若感不适,呼叫解毒中心/医生。漱口。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4-羟基-3-亚硝基-1-萘磺酸水合物
百分比: >98.0%(T)
CAS编码: 3682-32-4
俗名: 2-Nitroso-1-naphthol-4-sulfonic Acid Hydrate
分子式:
C10H7NO5S·xH2O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 一旦发生火警,有爆炸的危险。由于爆炸的危险,远距离灭火。
小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。切勿引起泄漏、溢出或分散。切勿产
生不必要的蒸气。远离热源/火花/明火/热表面。禁烟。采取措施防止静电积累。使用
防爆设备。避免冲击和摩擦。处理后彻底清洗双手和脸。
4-羟基-3-亚硝基-1-萘磺酸水合物 修改号码:6
模块 7. 操作处置与储存
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
确保容器不会受到未测影响,比如跌落或脱落。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 红黄色-深黄红色
气味: 无资料
pH: 无数据资料
熔点: 145°C (分解)
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 热、撞击、摩擦等条件下可能爆炸分解。
避免接触的条件: 热, 冲击, 摩擦
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
4-羟基-3-亚硝基-1-萘磺酸水合物 修改号码:6
模块 11. 毒理学信息
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门和专家。建议在可燃溶剂中溶解混合,然后在装有后燃和洗涤装置的化
学焚烧炉中慢慢焚烧。如果一次性焚烧大量物质,可能发生爆炸。废弃处置时请遵守国家、地区和当地的所有法规
。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 4-Hydroxy-3-nitroso-1-naphthalenesulfonic Acid Hydrate
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第4级
急性毒性(经皮) 第4级
急性毒性(吸入) 第4级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 吸入或皮肤接触或吞咽有害。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
4-羟基-3-亚硝基-1-萘磺酸水合物 修改号码:6
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。若感不适,呼叫解毒
中心/医生。
食入:若感不适,呼叫解毒中心/医生。漱口。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4-羟基-3-亚硝基-1-萘磺酸水合物
百分比: >98.0%(T)
CAS编码: 3682-32-4
俗名: 2-Nitroso-1-naphthol-4-sulfonic Acid Hydrate
分子式:
C10H7NO5S·xH2O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 一旦发生火警,有爆炸的危险。由于爆炸的危险,远距离灭火。
小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。切勿引起泄漏、溢出或分散。切勿产
生不必要的蒸气。远离热源/火花/明火/热表面。禁烟。采取措施防止静电积累。使用
防爆设备。避免冲击和摩擦。处理后彻底清洗双手和脸。
4-羟基-3-亚硝基-1-萘磺酸水合物 修改号码:6
模块 7. 操作处置与储存
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
确保容器不会受到未测影响,比如跌落或脱落。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 红黄色-深黄红色
气味: 无资料
pH: 无数据资料
熔点: 145°C (分解)
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 热、撞击、摩擦等条件下可能爆炸分解。
避免接触的条件: 热, 冲击, 摩擦
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
4-羟基-3-亚硝基-1-萘磺酸水合物 修改号码:6
模块 11. 毒理学信息
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门和专家。建议在可燃溶剂中溶解混合,然后在装有后燃和洗涤装置的化
学焚烧炉中慢慢焚烧。如果一次性焚烧大量物质,可能发生爆炸。废弃处置时请遵守国家、地区和当地的所有法规
。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-氨基-3-羟基-1-萘磺酸 2-amino-1-naphthol-4-sulfonic acid 567-13-5 C10H9NO4S 239.252
反应信息
-
作为反应物:描述:2-亚硝基-1-萘酚-4-磺酸水合物 在 platinum on activated charcoal 、 氢气 作用下, 以 乙醇 为溶剂, 60.0~100.0 ℃ 、4.0 MPa 条件下, 以92.5%的产率得到4-氨基-3-羟基-1-萘磺酸参考文献:名称:一种催化加氢制备氨基萘酚磺酸的方法摘要:本发明公开了一种催化加氢制备氨基萘酚磺酸的方法,属于有机合成技术领域。该方法是以雷尼镍、Pt/C或Pd/C为催化剂,加入的催化剂量为加入的亚硝基或硝基萘酚磺酸质量的0.5~8%,于50~120℃下,催化加氢反应的时间为0.5~5小时,加氢反应压力为0.5~4 MPa,制得氨基萘酚磺酸。产品液相色谱含量98%以上,收率大于90%。本发明工艺操作简便,对设备无腐蚀,反应完成后产品无需后处理可直接用于下步反应,不产生任何固体和液体废弃物,避免了保险粉工艺废固处理困难和污染问题。应用本工艺获得的产品质量好,是一种环境友好型绿色生产工艺。公开号:CN105777588A
-
作为产物:描述:参考文献:名称:Rieche,A.; Seeboth,H., Justus Liebigs Annalen der Chemie, 1960, vol. 638, p. 57 - 66摘要:DOI:
文献信息
-
Influence of anionic sulfonate-containing co-ligands on the solid structures of silver complexes supported by 4,4′-bipyridine bridges作者:Hua Wu、Xian-Wu Dong、Hai-Yan Liu、Jian-Fang Ma、Shun-Li Li、Jin Yang、Ying-Ying Liu、Zhong-Min SuDOI:10.1039/b806195e日期:——In this paper, ten new silver compounds, namely [Ag(bipy)](L1)·H2O (1), [Ag(bipy)](L2)·2H2O (2), [Ag2(bipy)2(H2O)2](L3)·H2O (3), [Ag(L4)(bipy)]·H2O (4), [Ag(L5)(bipy)] (5), [Ag(L6)(bipy)]·0.5CH3CN (6), [Ag3(L7)2(bipy)2]·2(H2O) (7), [Ag2(L8)(bipy)1.5(H2O)]·H2O (8), [Ag2(L9)(bipy)2(H2O)2] (9) and [Ag3(L10)(bipy)2][(bipy)(H2O)2]·(H2O)3.5 (10) (where bipy = 4,4â²-bipyridine, L1 = 6-amino-1-naphthalenesulfonate anion, L2 = 2-naphthalenesulfonate anion, L3 = sulfosalicylate anion, L4 = p-aminobenzenesulfonate anion, L5 = 4-dimethyaminoazobenzenen-4â²-sulfonate anion, L6 = 2,5-dichloro-4-amino-benzenesulfonate anion, L7 = 8-hydroxyquinoline-5-sulfonate anion, L8 = 2-nitroso-1-naphthol-4-sulfonate anion, L9 = 2,6-naphthalenedisulfonate anion and L10 = 1,3,5-naphthalenetrisulfonate anion), have been synthesized and characterized by elemental analyses, IR spectroscopy and X-ray crystallography. In compounds 1â6, Ag(I) centers are linked by bipy ligands to form 1D Agâbipy chain structures, in which the sulfonate anions of compounds 1â3 act as counter ions. The sulfonate anions of compounds 4 and 5 connect Agâbipy chains to form 1D double chain structures, respectively. The sulfonate anions of compound 6 connect Agâbipy chains to form a 2D layer structure. Unexpectedly, compound 7 shows a hinged chain structure, and these chains interlace with each other through hydrogen bonds and ÏâÏ interactions to generate a 3D structure with channels along the c axis. Compounds 8 and 9 show 1D ladder-like structures. In compound 10, the Agâbipy chains are connected by sulfonate anions to generate a 3D poly-threaded network, in which an isolated Agâbipy chain is inserted. The results indicate that the anionic sulfonate-containing co-ligands play an important role in the final structures of the Ag(I) complexes. Additionally, the luminescent properties of these compounds were also studied.在本文中,合成并表征了十种新的银化合物,分别为[Ag(bipy)](L1)·H2O (1),[Ag(bipy)](L2)·2 (2),[Ag2(bipy)2( )2](L3)· (3),[Ag(L4)(bipy)]· (4),[Ag(L5)(bipy)] (5),[Ag(L6)(bipy)]·0.5CH3CN (6),[Ag3(L7)2(bipy)2]·2( ) (7),[Ag2(L8)(bipy)1.5( )]· (8),[Ag2(L9)(bipy)2( )2] (9) 和 [Ag3(L10)(bipy)2][(bipy)( )2]·( )3.5 (10)(其中bipy = 4,4′-联吡啶,L1 = 6-氨基-1-萘磺酸根离子,L2 = 2-萘磺酸根离子,L3 = 磺基水杨酸根离子,L4 = 对氨基苯磺酸根离子,L5 = 4-二甲基氨基偶氮苯-4′-磺酸根离子,L6 = 2,5-二氯-4-氨基苯磺酸根离子,L7 = 8-羟基喹啉-5-磺酸根离子,L8 = 2-亚硝基-1-萘酚-4-磺酸根离子,L9 = 2,6-萘二磺酸根离子,L10 = 1,3,5-萘三磺酸根离子),这些化合物通过元素分析、红外光谱和X射线晶体学进行了表征。在化合物1-6中,银(I)中心通过bipy配体连接形成一维Ag-bipy链结构,其中化合物1-3的磺酸根离子作为对离子。化合物4和5的磺酸根离子分别连接Ag-bipy链形成一维双链结构。化合物6的磺酸根离子连接Ag-bipy链形成二维层状结构。意外的是,化合物7显示出一个铰链链结构,这些链通过氢键和π-π相互作用交织在一起,生成一个沿c轴的三维结构,并形成通道。化合物8和9显示出一维梯形结构。在化合物10中,Ag-bipy链通过磺酸根离子连接形成三维聚线程网络,其中一个孤立的Ag-bipy链被插入其中。结果表明,含磺酸根的阴离子共配体在Ag(I)配合物的最终结构中起着重要作用。此外,这些化合物的发光特性也进行了研究。
-
Influence of anionic sulfonate-containing and nitrogen-containing mixed-ligands on the structures of silver coordination polymers作者:Hua Wu、Xian-Wu Dong、Jian-Fang Ma、Hai-Yan Liu、Jin Yang、Hong-Ye BaiDOI:10.1039/b814942a日期:——In this article, a series of mixed-ligand Ag(I) coordination polymers, namely, Ag(L1)H2O (1), Ag2(hmt)2(L1)2 (2), [Ag(HL2)]H2O (3), [Ag(L2)(biim)][Ag(biim)] (4), [K5(L3)2][Ag(H2O)2] (5), [Ag2(L3)(3-iso)][Ag(3-iso)]5H2O (6), [Ag3(L3)(bipy)3(H2O)]6H2O (7), [Ag3(L3)(biim)3]H2O (8), and [Ag3(L3)(hmt)2(H2O)3]H2O (9) (where hmt = hexamethylenetetramine, bipy = 4,4′-bipyridine, biim = 1,1′-(1,4-butanediyl)-bis(imidazole), 3-iso = 3-methylisoquinoline, L1 = 2,5-dichloro-4-amino-benzenesulfonate anion, L2 = 2-nitroso-1-naphthol-4-sulfonate anion and L3 = 4-hydroxy-5-nitro-1,3-benzenedisulfonate anion), have been synthesized by varying the anionic sulfonate-containing ligands and nitrogen-containing secondary ligands. Compounds 2 and 4 were synthesized through their parent compounds 1 and 3, respectively, while 6–9 were obtained by their parent compound 5. Ag(I) centers of 1 are linked by L1 anions to generate a 1D polymeric chain, while Ag(I) centers of 2 are bridged by hmt ligands to form a 2D polymeric layer. In 3, Ag(I) centers are connected by L2 anions to yield a 1D double chain, whereas Ag(I) centers in 4 are joined by biim ligands and L2 anions, generating an unusual anionic chain and a cationic chain, respectively. In 5, Ag(I) centers are coordinated by water molecules, and K(I) cations are coordinated by L3 anions to form a pillared layered structure with inorganic layers and organic pillars. In 6, Ag(I) centers are coordinated by 3-iso ligands and L3 anions to generate a discrete molecular structure. In 7, bipy ligands bridge Ag(I) centers to give Ag–bipy chains, which are further linked by L3 anions to produce a 2D polymeric layer. In 8, Ag(I) centers are bridged by biim ligands to generate a 1D polymeric chain. In 9, the hmt ligands connect Ag(I) centers to give a binodal (3,4)-connected net with (63)(65.8)2 topology. These results indicate that anionic sulfonate-containing ligands and the nitrogen-containing secondary ligands play important roles in the structural formation of the Ag(I) complexes. Additionally, the luminescent properties, elemental analyses and IR spectroscopy of these compounds were also studied.在本文中,合成了一系列混合配体的Ag(I)配位聚合物,即Ag(L1)H2O (1)、Ag2(hmt)2(L1)2 (2)、[Ag(HL2)] (3)、[Ag(L2)(biim)][Ag(biim)] (4)、[K5(L3)2][Ag( )2] (5)、[Ag2(L3)(3-iso)][Ag(3-iso)]·5 (6)、[Ag3(L3)(bipy)3( )]·6 (7)、[Ag3(L3)(biim)3]· (8)和[Ag3(L3)(hmt)2( )3]· (9)(其中hmt = 六甲基四胺,bipy = 4,4′-联吡啶,biim = 1,1′-(1,4-丁烷二基)-双(咪唑),3-iso = 3-甲基异喹啉,L1 = 2,5-二氯-4-氨基苯磺酸根阴离子,L2 = 2-亚硝基-1-萘醇-4-磺酸根阴离子,L3 = 4-羟基-5-硝基-1,3-苯二磺酸根阴离子),通过改变含磺酸根的阴离子配体和含氮的二级配体合成。化合物2和4分别通过其母体化合物1和3合成,而化合物6–9则通过其母体化合物5获得。化合物1中,Ag(I)中心通过L1阴离子连接,形成一维聚合物链;而在化合物2中,Ag(I)中心通过hmt配体桥接,形成二维聚合物层。在化合物3中,Ag(I)中心由L2阴离子连接,生成一维双链,而在化合物4中,Ag(I)中心由biim配体和L2阴离子连接,分别生成不寻常的阴离子链和阳离子链。在化合物5中,Ag(I)中心与水分子配位,K(I)阳离子与L3阴离子配位,形成一种带有无机层和有机柱的立柱结构。在化合物6中,Ag(I)中心与3-iso配体和L3阴离子配位,生成离散的分子结构。在化合物7中,bipy配体桥接Ag(I)中心,形成Ag–bipy链,这些链又通过L3阴离子进一步连接,产生二维聚合物层。在化合物8中,Ag(I)中心由biim配体桥接,生成一维聚合物链。在化合物9中,hmt配体连接Ag(I)中心,形成具有(63)(65.8)2拓扑结构的双节点(3,4)连接网。这些结果表明,含磺酸根的阴离子配体和含氮的二级配体在Ag(I)配合物的结构形成中起着重要作用。此外,还研究了这些化合物的发光性质、元素分析和红外光谱。
-
[DE] VERFAHREN ZUR HERSTELLUNG VON CARBOXYRESTE AUFWEISENDEN ORGANOSILICIUMVERBINDUNGEN<br/>[EN] METHOD FOR THE PRODUCTION OF ORGANOSILICON COMPOUNDS COMPRISING CARBOXY RADICALS<br/>[FR] PROCEDE POUR PRODUIRE DES COMPOSES D'ORGANOSILICIUM PRESENTANT DES RADICAUX CARBOXY申请人:WACKER CHEMIE GMBH公开号:WO2005103060A1公开(公告)日:2005-11-03Beschrieben wird ein Verfahren zur Herstellung von Carbonylreste aufweisenden Organosiliciumverbindungen (2) durch Oxidation von Carbinolreste-aufweisenden Organosiliciumverbindungen (1) mit Hilfe eines Mediators (3) ausgewählt aus der Gruppe der aliphatischen, cycloaliphatischen, heterocyclischen und aromatischen NO-, NOH- und (I)-haltigen Verbindungen und eines Oxidationsmittels (4), mit der Maßgabe, dass die Reaktion unter ständiger Kontrolle des pH-Wertes bei einem pH ≥ 3 durchgeführt wird und dass die Carbinolreste in den eingesetzten Organosiliciumverbindungen (1) vorwiegend zu Carboxyresten oxidiert werden.
-
POLYMERIZABLE COMPOSITION FOR COLOR FILTER, COLOR FILTER, AND SOLID-STATE IMAGING DEVICE申请人:Shimada Kazuto公开号:US20110217637A1公开(公告)日:2011-09-08A polymerizable composition for a color filter, including (A) a polymerizable compound, (B) a polymerization initiator, (C) a coloring agent, and (D) a polymer including at least a group having polymerization inhibiting ability and a group having surface localizability.
-
Verfahren zur Herstellung aromatischer Aminohydroxyverbindungen申请人:BAYER AG公开号:EP0001982A1公开(公告)日:1979-05-30Die Erfindung betrifft ein Verfahren zur Herstellung von Aminohydroxyverbindungen durch Umsetzung aromatischer Nitrosoverbindungen mit Hydrazin, bei dem man die aromatische Nitrosoverbindung und das Hydrazin in wäßrigem Medium bei Temperaturen von 10 bis 50° C in etwa äquivalenten Mengen umsetzt, wobei man die aromatische Nitrosoverbindung vorlegt und das Hydrazin zugibt.
表征谱图
-
氢谱1HNMR
-
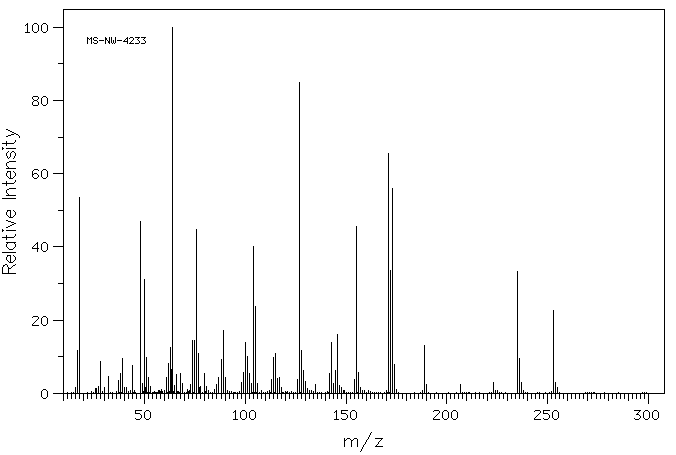
质谱MS
-
碳谱13CNMR
-
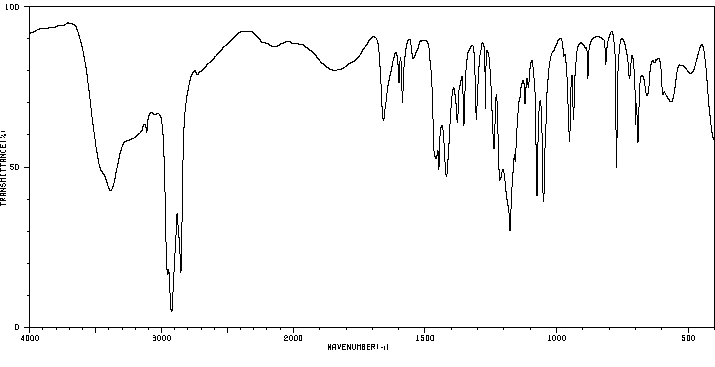
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮