trans-1,4-di(4-methylphenyl)-2,5-diphenylpiperazine | 33169-39-0
中文名称
——
中文别名
——
英文名称
trans-1,4-di(4-methylphenyl)-2,5-diphenylpiperazine
英文别名
2,5-diphenyl-1,4-di-p-tolyl-piperazine;1,4-Bis(4-methylphenyl)-2,5-diphenylpiperazine
CAS
33169-39-0
化学式
C30H30N2
mdl
——
分子量
418.582
InChiKey
ULOYGMQTSVKBAO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:611.8±55.0 °C(Predicted)
-
密度:1.107±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):7.4
-
重原子数:32
-
可旋转键数:4
-
环数:5.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:6.5
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为产物:描述:N-(p-tolyl)-1-phenyl-2-aminoethanol 在 sodium hydroxide 、 四丁基硫酸氢铵 、 对甲苯磺酰氯 作用下, 以 水 、 苯 为溶剂, 反应 0.5h, 以70%的产率得到N-(4-methylphenyl)-2-phenyl-aziridine参考文献:名称:A FACILE SYNTHESIS OF N-ARYL AZIRIDINES摘要:Reaction of N-aryl-beta -amino alcohols with p-toluenesulphonyl chloride under phase transfer catalytic condition gave the corresponding N-aryl aziridines in good yields, whereas N-alkyl-beta -amino alcohol [for e.g., L-ephedrine] gave the corresponding N-tosyl derivative as the major product, along with the expected N-alkyl aziridines in lower yield.DOI:10.1081/scc-100103544
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
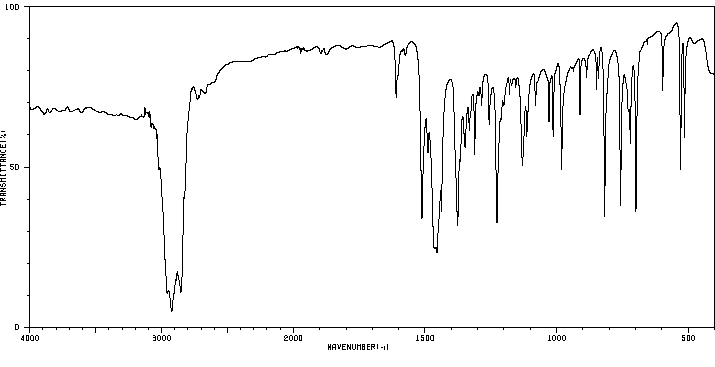
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2S)-4-[7-(8-氯-1-萘)-5,6,7,8-四氢-2-[[((2S)-1-甲基-2-吡咯烷基]甲氧基]吡啶基[3,4-d]嘧啶-4-基]-1-(2-氟-1-氧代-2-丙烯-1-基)-2-哌Chemicalbook嗪乙腈;2-((S)-4-(7-(8-氯萘-1-基)-2-((((S)-1-
齐拉西酮相关物质C
齐拉西酮杂质E
齐拉西酮开环物,氨基酸杂质
齐拉西酮亚砜
齐拉西酮 盐酸盐 一水合物
齐拉西酮
鲸蜡硬脂醇
鲁拉西酮杂质3
鲁拉西酮杂质23
鲁拉西酮杂质14
鲁拉西酮杂质11
鲁拉西酮
鲁拉西杂质E
鲁拉西杂质1
高分子量聚合三嗪类无卤阻燃剂
驱虫灵D
马福拉嗪
马来酸阿伐曲泊帕
顺式-1-乙酰基-2,6-二甲基-4-亚硝基-哌嗪
雷诺嗪双(N-氧化物)
陶扎色替
阿达色林
阿莫西林二氧代哌嗪
阿立哌唑羟基丁基杂质
阿立哌唑杂质26
阿立哌唑USP相关物质H
阿立哌唑N4-氧化物
阿立哌唑N,N-二氧化物
阿立哌唑EP杂质D
阿立哌唑-d8
阿立哌唑
阿泊替尼
阿替韦啶
阿拉诺丁
阿扎哌醇
阿得巴司
阿尔哌汀
阿伐曲泊帕杂质
间羟基苯基哌嗪
钾 1-甲基-4-三氟硼酸三甲基哌嗪
钠4-(4-乙酰基-1-哌嗪基)苯酚
酮齐拉西酮
酮酮唑油酸酯
酚酞单磷酸酯二-(2-氨基-2-甲基-1,3-丙二醇)盐
选择性氟试剂II
达鲁舍替
达哌唑
赫普索
赤霉素A7甲酯