2-chloro-7-phenyltropone | 90128-01-1
中文名称
——
中文别名
——
英文名称
2-chloro-7-phenyltropone
英文别名
2-Chlor-7-phenyl-tropon;2,4,6-Cycloheptatrien-1-one, 2-chloro-7-phenyl-;2-chloro-7-phenylcyclohepta-2,4,6-trien-1-one
CAS
90128-01-1
化学式
C13H9ClO
mdl
——
分子量
216.667
InChiKey
GSMAZDFUYTUQTD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:75 °C(Solv: ethanol (64-17-5))
-
沸点:389.6±42.0 °C(Predicted)
-
密度:1.24±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:15
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Reaction of 2-Chloro-3-phenyltropone and 2-Chloro-7-phenyltropone with Ammonia and Amines摘要:DOI:10.1246/bcsj.32.272
-
作为产物:参考文献:名称:Muroi, Nippon Kagaku Zasshi, 1959, vol. 80, p. 185,186摘要:DOI:
文献信息
-
The Grignard Reaction of 3-Halotropolone Methyl Ethers作者:Haruki Tsuruta、Toshio MukaiDOI:10.1246/bcsj.41.2489日期:1968.10The Grignard reaction of the methyl ethers (I and III) of 3-bromotropolone and those (II and IV) of 3-chlorotropolone with methylmagnesium iodide or phenylmagnesium bromide were investigated. When I or II was allowed to react with methylmagnesium iodide, 2-methoxyphenyldimethylcarbinol (V) and 2-methoxy-6-isopropylphenol (VI) were obtained. The reaction of I or II with phenylmagnesium bromide afforded
-
Synthesis, Chiroptical Properties and Density Functional Theory Calculations of 3,3’-Biphenyl-2,2’-BiTropone作者:Marino Cavazza、Mario Cifelli、Valentina Domenici、Tiziana Funaioli、Benedetta Mennucci、Carlo Alberto Veracini、Maurizio ZandomeneghiDOI:10.1002/chir.22191日期:2013.10The synthesis of new bitropone derivatives, namely, 3,3'‐biphenyl‐2,2'‐bitropone and 7,7’‐biphenyl‐2,2'‐bitropone, are reported. Isolation of enantiomers arising from restricted rotation around the C‐C bond connecting the tropone moieties was attempted by means of chiral high performance liquid chromatography (HPLC). No separation was obtained for 7,7’‐biphenyl‐2,2'‐bitropone. For 3,3'‐biphenyl‐2,2'‐bitropone据报道,合成了新的Bitropone衍生物,即3,3'-Biphenyl-2,2'-Bitropone和7,7'-Biphenyl-2,2'-Bitropone。尝试通过手性高效液相色谱(HPLC)分离由于围绕托康酮部分的C‐C键旋转受限而产生的对映异构体。没有分离出7,7'-联苯-2,2'-苯丁二酮。对于3,3'-Biphenyl-2,2'-Bitropone,遇到困难的原因是峰的分离系数低,并且存在快速消旋过程。但是,通过将圆二色性(CD)光谱仪和UV-vis分光光度检测器串联到HPLC仪器上,可以获得对映体的定量手性数据。CD和UV-vis光谱在绝对构象方面的分析是在密度泛函理论(DFT)级别进行的理论计算的帮助下进行的。获得了处于基态的最稳定的3,3'-联苯-2,2'-二氢吡喃酮构象。从这些最小的能量构象开始,有可能计算出与实验的光谱非常吻合的理论CD和UV吸收光谱。通过该比较,确
-
Cavazza, Marino; Morganti, Gioia; Guerriero, Antonio, Journal of the Chemical Society. Perkin transactions I, 1984, # 2, p. 199 - 202作者:Cavazza, Marino、Morganti, Gioia、Guerriero, Antonio、Pietra, FrancescoDOI:——日期:——
-
CAVAZZA, M.;MORGANTI, G.;GUERRIERO, A.;PIETRA, F., J. CHEM. SOC. PERKIN TRANS., 1984, N 2, 199-202作者:CAVAZZA, M.、MORGANTI, G.、GUERRIERO, A.、PIETRA, F.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
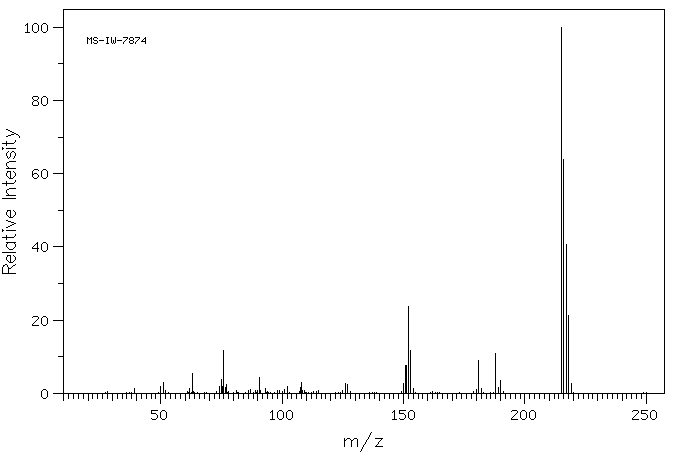
质谱MS
-
碳谱13CNMR
-
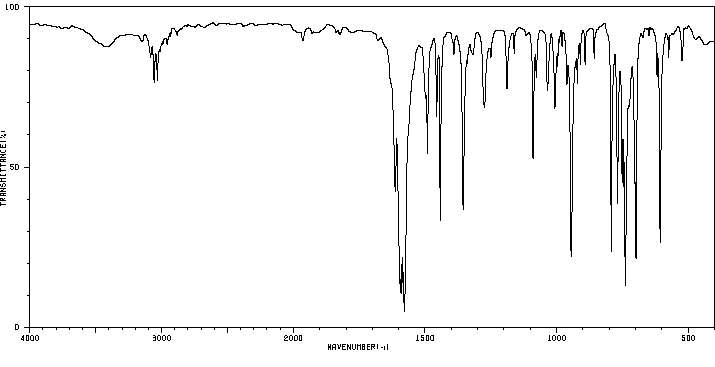
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
脱羰秋水仙碱
红陪酚四甲基醚
红倍酚
秋水仙碱甲硫代磺酸盐
秋水仙碱
硫代秋水仙碱
甲基丙烯酸7-氧代-4-(苯基偶氮)-1,3,5-环庚三烯-1-基酯
甲基6-肼基-7-氧代-1,3,5-环庚三烯-1-羧酸酯
环庚三烯酮
环庚三烯酚酮
氨甲酸,(1-乙基戊基)-,甲基酯(9CI)
桧木醇
异秋水仙胺
尼楚酮
对二硫辛酸
双环[4.4.1]十一碳-1(10),2,4,6,8-五烯-11-酮
双环[4.1.0]庚-1,3,5-三烯-7-酮
去乙酰氨基秋水仙碱
原秋水仙碱
十四烷酸,4-(十八烷氧基)-7-羰基-1,3,5-环庚三烯-1-基酯
乙基[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]氨基甲酸酯
三甲基秋水仙素酸
三甲基秋水仙素酸
三(2-羟基-2,4,6-环庚三烯-1-酮)-铟
α-(异丙基)-γ,γ-二甲基环己丙醇
beta-斧松素
[(7S)-7-乙酰氨基-1,3-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-2-基]2-氯乙酸酯
[(7S)-7-乙酰氨基-1,2-二甲氧基-10-甲硫基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-3-基]2-氯乙酸酯
N-(2-巯基乙基)秋水仙胺
N-脱乙酰基3-去甲基硫代秋水仙碱
N-脱乙酰基,1,2,3,10-脱甲基秋水仙碱
N-甲酰脱乙酰秋水仙碱
N-甲酰基秋水仙胺
N-甲基-秋水仙碱
N-三氟乙酰基-N-甲基-去乙酰基秋水仙碱
N-[(S)-5,6,7,9-四氢-1,2,3,10-四甲氧基-9-氧代苯并[a]庚搭烯-7-基]-2,2,2-三氟乙酰胺
N-[(7S)-4-(羟基甲基)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-10-(丁基氨基)-5,6,7,9-四氢-1,2,3-三甲氧基-9-氧代苯并[a]庚搭烯-7-基]-乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲硫基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3-三甲氧基-9-氧代-10-(苯基甲基氨基)-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-[(7S)-1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基]丙酰胺
N-[(7R)-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基]乙酰胺
N-(乙氧基乙酰基)去乙酰基硫代秋水仙碱
N-(5,6,7,9-四氢-1,2,3-三甲氧基-10-甲硫基-9-氧代苯并[a]庚搭烯-7-基)氨基甲酸乙酯
N-(4-甲酰基-1,2,3,10-四甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(10-二甲基氨基-1,2,3-三甲氧基-9-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,9-四甲氧基-10-氧代-6,7-二氢-5H-苯并[d]庚搭烯-7-基)乙酰胺
N-(1,2,3,10-四甲氧基-9-氧代-5,6,7,9-四氢苯并[a]庚搭烯-7-基)乙酰胺
9H-三苯并[A,C,E][7]环轮烯-9-酮
8H-环庚三烯并[c]异噻唑-8-酮,3-甲基-