3-氨基-5,6-二苯基-1,2,4-三嗪 | 4511-99-3
中文名称
3-氨基-5,6-二苯基-1,2,4-三嗪
中文别名
——
英文名称
5,6-diphenyl-1,2,4-triazine-3-amine
英文别名
5,6-diphenyl-1,2,4-triazin-3-amine
CAS
4511-99-3
化学式
C15H12N4
mdl
MFCD00047461
分子量
248.287
InChiKey
NZRHOWNFGASHMN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:177 °C
-
沸点:463.6±48.0 °C(Predicted)
-
密度:1.229±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:19
-
可旋转键数:2
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:64.7
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2933699090
-
储存条件:室温
SDS
| Name: | 5 6-Diphenyl-1 2 4-triazin-3-amine 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 4511-99-3 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 4511-99-3 | 5,6-Diphenyl-1,2,4-triazin-3-amine | 97% | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 4511-99-3: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: cream
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 172 - 174 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C15H12N4
Molecular Weight: 248
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Acids, oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 4511-99-3: XY3177800 LD50/LC50:
Not available.
Carcinogenicity:
5,6-Diphenyl-1,2,4-triazin-3-amine - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 4511-99-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 4511-99-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 4511-99-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3-azido-5,6-diphenyl-1,2,4-triazine 88281-91-8 C15H10N6 274.285 3-氯-5,6-二苯基-1,2,4-三嗪 3-chloro-5,6-diphenyl-1,2,4-triazine 34177-11-2 C15H10ClN3 267.717 3-氨基-5-苯基-1,2,4-三嗪 5-phenyl-1,2,4-triazin-3-amine 942-60-9 C9H8N4 172.189 —— 6-bromo-5-phenyl-1,2,4-triazin-3-amine 1321517-51-4 C9H7BrN4 251.085 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-(2-吡啶基)-5,6-二苯基-1,2,4-三嗪 5,6-diphenyl-1,2,4-triazine 21134-91-8 C15H11N3 233.272 —— 3-Diacetylamino-5,6-diphenyl-as-triazin 4512-05-4 C19H16N4O2 332.362 3-氯-5,6-二苯基-1,2,4-三嗪 3-chloro-5,6-diphenyl-1,2,4-triazine 34177-11-2 C15H10ClN3 267.717 —— 2,3,6-triphenyl-imidazo[1,2-b][1,2,4]triazine 10243-70-6 C23H16N4 348.407 —— 1,4-bis-(2,3-diphenyl-imidazo[1,2-b][1,2,4]triazin-6-yl)-benzene —— C40H26N8 618.7 —— 6-(Biphenyl-4-yl)-2,3-diphenylimidazo[1,2-b][1,2,4]triazine —— C29H20N4 424.5 2,3,6,7-四苯基咪唑并[1,2-b][1,2,4]三嗪 2,3,6,7-tetraphenyl-imidazo[1,2-b][1,2,4]triazine 70111-81-8 C29H20N4 424.505 —— 6-(3-Methoxyphenyl)-2,3-diphenylimidazo[1,2-b][1,2,4]triazine —— C24H18N4O 378.4 —— 2,3,7-Triphenyl-6-(4-phenylphenyl)imidazo[1,2-b][1,2,4]triazine 94103-65-8 C35H24N4 500.6
反应信息
-
作为反应物:描述:参考文献:名称:一些含有 1,2,4-三嗪部分的新型巴比妥酸和硫代巴比妥酸及其相关体系作为除草剂的合成摘要:为了寻找具有高生物活性的巴比妥酸和硫代巴比妥酸衍生物,通过将异氰酸酯和异硫氰酸酯加成到 3-氨基- 5,6-二苯基-1,2,4-三嗪 (1) 然后与丙二酸二乙酯发生闭环反应。此外,相关系统的化学反应性是通过巴比妥酸和硫代巴比妥酸衍生物与芳香醛和/或氟酰化反应的缩合获得的。从元素分析和光谱数据(IR、1H NMR、13C NMR 和 MS)推导出所有产物的结构。还评估了产品的除草活性。DOI:10.1155/2019/3035107
-
作为产物:描述:3-(甲基硫代)-5-苯基-1,2,4-三嗪 在 四(三苯基膦)钯 N-溴代丁二酰亚胺(NBS) 、 氨 、 potassium carbonate 、 ferric nitrate 、 间氯过氧苯甲酸 作用下, 以 四氢呋喃 、 1,4-二氧六环 、 二氯甲烷 、 水 、 N,N-二甲基甲酰胺 为溶剂, 反应 10.25h, 生成 3-氨基-5,6-二苯基-1,2,4-三嗪参考文献:名称:[EN] 1,2,4-TRIAZINE-4-AMINE DERIVATIVES
[FR] DÉRIVÉS DE 1,2,4-TRIAZINE-4-AMINE摘要:根据该发明提供了一种A1式化合物,可能对通过抑制A1-A2b或特别是A2a受体而得到改善的疾病或紊乱治疗有用,其中A1式化合物具有以下结构,其中,A代表Cy1或HetA;Cy1代表一个由一个、两个或三个环组成的5-至14-成员芳香、完全饱和或部分不饱和的碳环系统,该Cy1基团可选择地被一个或多个R4a取代基所取代;HetA代表一个由一个、两个或三个环组成的5-至14-成员杂环基团,可能是芳香、完全饱和或部分不饱和的,并且含有从O、S和N中选择的一个或多个杂原子,该杂环基团可能包括一个、两个或三个环,且该HetA基团可选择地被一个或多个R4b取代基所取代;B代表Cy2或HetB;Cy2代表一个由一个或两个环组成的3-至10-成员芳香、完全饱和或部分不饱和的碳环系统,该Cy2基团可选择地被一个或多个R4c取代基所取代;HetB代表一个由一个或两个环组成的3-至10-成员杂环基团,可能是芳香、完全饱和或部分不饱和的,并且含有从O、S和N中选择的一个或多个杂原子,该杂环基团可能包括一个或两个环,且该HetB基团可选择地被一个或多个R4d取代基所取代。公开号:WO2011095625A1
文献信息
-
Studies in the triazine series including a new synthesis of 1:2:4-triazines作者:P.V. Laakso、R. Robinson、H.P. VandrewalaDOI:10.1016/0040-4020(57)85014-5日期:1957.1mono-aroylhydrazones of benzil are cyclised by ammonium acetate in hot acetic acid to tri-substituted-1:2:4-triazines. The yield is favourable and it is not necessary, or even advantageous, to isolate the presumed intermediates. The new synthesis has been applied to a sufficient range of examples to establish its status as a general method. In the case of phenanthraquinone the reaction took a more complex course and
-
Deamination of 2-Aminothiazoles and 3-Amino-1,2,4-triazines with Nitric Oxide in the Presence of a Catalytic Amount of Oxygen.作者:Takashi ITOH、Yuji MATSUYA、Kazuhiro NAGATA、Akio OHSAWADOI:10.1248/cpb.45.1547日期:——2-Aminothiazoles, 2-aminobenzazoles, and 3-amino-1, 2, 4-triazines were deaminated using nitric oxide (NO) in the presence of a catalytic amount of oxygen to afford corresponding unsubstituted heterocycles in good yields.
-
Synthesis, spectroscopic (UV–vis and GIAO NMR), crystallographic and theoretical studies of triazine heterocyclic derivatives作者:Salman A. Khan、Abdullah Y. Obaid、Laila M. Al-Harbi、Muhammad Nadeem Arshad、Onur Şahin、Cem Cüneyt Ersanlı、R.M. Abdel-Rehman、Abdullah M. Asiri、Michael B. HursthouseDOI:10.1016/j.molstruc.2015.04.036日期:2015.9Abstract This work presents the synthesis and characterization of triazine heterocyclic derivatives. The spectroscopic properties like nuclear magnetic resonance [NMR, (1H and 13C)] were recorded in CDCl3 solution and Ultraviolet–Visible (UV–vis) absorption spectrums of compounds, 5,6-diphenyl-[1,2,4]triazin-3-ylamine (1), (5,6-diphenyl-[1,2,4]triazin-3-yl)-hydrazine (2) and 5,6-diphenyl-4H-[1,2,4]摘要 这项工作介绍了三嗪杂环衍生物的合成和表征。在CDCl3 溶液中记录了核磁共振[NMR, (1H and 13C)] 等光谱特性和化合物5,6-二苯基-[1,2,4]triazin-的紫外-可见(UV-vis)吸收光谱- 3-基胺(1)、(5,6-二苯基-[1,2,4]三嗪-3-基)-肼(2)和5,6-二苯基-4H-[1,2,4]三嗪- 3-硫酮 (3) 在 200–800 nm 范围内记录,使用氯仿作为基础溶剂。使用密度泛函理论 (DFT) 和 6-31G(d,p) 基组计算了具有基态三嗪杂环衍生物的化合物的分子几何结构,并与 X 射线实验数据进行了比较。计算结果表明优化后的几何结构可以很好地再现晶体结构。总静态偶极矩(μ),已使用相同方法计算了所研究分子的平均线性极化率 (α) 和第一超极化率 (β) 值。已使用 B3LYP 方法通过应用极化连续模型 (PCM) 和 6-31G(d
-
유기 화합물 및 이를 이용한 유기 전계 발광 소자申请人:Solus Advanced Materials co., Ltd. 솔루스첨단소재 주식회사(120190660630) Corp. No ▼ 214911-0058927BRN ▼668-81-01406公开号:KR20210078631A公开(公告)日:2021-06-29본 발명은 신규한 유기 화합물 및 이를 이용한 유기 전계 발광 소자에 대한 것으로, 보다 상세하게는 발광능, 전자수송능, 전기화학적 안정성, 열적 안정성 등이 우수한 유기 화합물 및 이를 하나 이상의 유기물층에 포함함으로써 발광효율, 구동 전압, 수명 등의 특성이 향상된 유기 전계 발광 소자에 대한 것이다.本发明涉及一种新的有机化合物及其用于有机电致发光器件,更详细地说,涉及一种有机化合物,具有出色的发光性能、电子传输性能、电化学稳定性、热稳定性等特性,并通过将其包含在一个或多个有机层中,以提高发光效率、驱动电压、寿命等特性的有机电致发光器件。
-
Discovery of 1,2,4-Triazine Derivatives as Adenosine A<sub>2A</sub> Antagonists using Structure Based Drug Design作者:Miles Congreve、Stephen P. Andrews、Andrew S. Doré、Kaspar Hollenstein、Edward Hurrell、Christopher J. Langmead、Jonathan S. Mason、Irene W. Ng、Benjamin Tehan、Andrei Zhukov、Malcolm Weir、Fiona H. MarshallDOI:10.1021/jm201376w日期:2012.3.8Potent, ligand efficient, selective, and orally efficacious 1,2,4-triazine derivatives have been identified using structure based drug design approaches as antagonists of the adenosine A2A receptor. The X-ray crystal structures of compounds 4e and 4g bound to the GPCR illustrate that the molecules bind deeply inside the orthosteric binding cavity. In vivo pharmacokinetic and efficacy data for compound
表征谱图
-
氢谱1HNMR
-
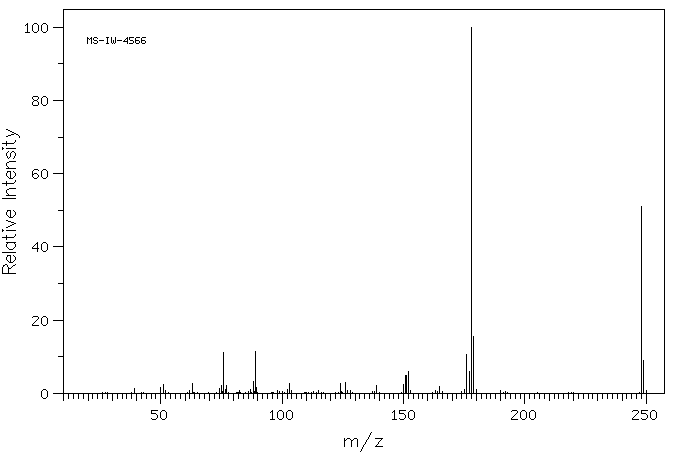
质谱MS
-
碳谱13CNMR
-
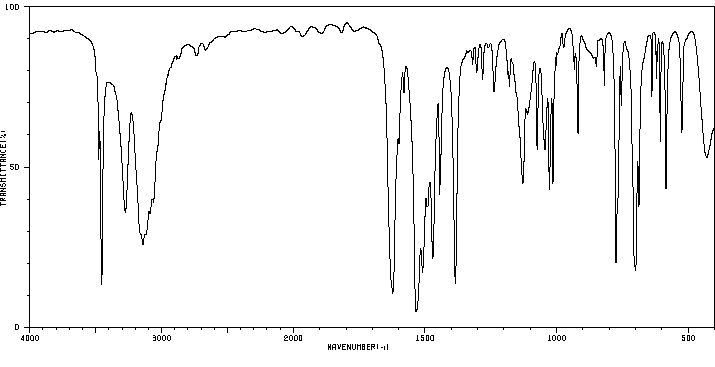
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿马诺嗪
阿特拉通
阿特拉津-乙氨基-15N1
阿特拉津-D5 同位素
阿特拉津
阿特拉嗪去异丙基-2-羟基
阿扎丙宗
达卡巴嗪相关物质B
败脂酸,丙-2-烯腈,苯乙烯
西草净亚砜
西草净
西玛津
螺拉秦
蜜勒胺
莠灭净
莠去津-特丁净混合物
莠去津-13C3
莠去津
草达津-2-羟基
草达津
苯酚,2-(4-氨基-6-乙氧基-1,3,5-三嗪-2-基)-
苯并呋喃,2-环丙基-
苯基-1,3,5-三嗪
苯嗪草酮-DESAMINO
苯嗪草酮
肼基氰尿酸盐
聚磷酸三聚氰胺
聚[[6-[(1,1,3,3-四甲基丁基)氨基]-1,3,5-三嗪-2,4-二基][(2,2,6,6-四甲基-4-哌啶基)亚氨基]-1,6-己二基[(2,2,6,6-四甲基-4-哌啶基)亚氨]]
聚(氧代-1,2-乙二氧基羰基-2,6-萘二基羰基)
羟硝基
美拉肼
美司钠EP杂质E
硫酸三聚氰胺
癸基-(二氯-[1,3,5]三嗪-2-基)-胺
甲氧丙净
甲基[2-(苯甲基氨基)-4-(4-氯苯基)-1,3-噻唑-5-基]乙酸酯
甲基6-甲基-1,2,3-三嗪-4-羧酸酯
甲基5-甲基-1,2,3-三嗪-4-羧酸酯
甲基-[1,2,4]噻嗪-3-基-胺
甲基(4Z)-4-(羟基亚胺)-2-甲基-4,5-二氢-1H-咪唑-1-羧酸酯
甲基(2E)-3-吖丙啶-1-基丙-2-烯酸酯
环氯胍硝酸盐
环氯胍
环己基三聚氰胺
环己基-(1-氧代-苯并[1,2,4]三嗪-3-基)-胺
环丙胺,N-[2-[(4-甲基苯基)硫代]乙基]-
环丙津-脱异丙基
环丙津-2-羟基
环丙津
环丙氨嗪-D4