3-苯基戊酸 | 5669-17-0
中文名称
3-苯基戊酸
中文别名
——
英文名称
3-phenylpentanoic acid
英文别名
2-phenylbutane carboxylic acid
CAS
5669-17-0
化学式
C11H14O2
mdl
MFCD01722151
分子量
178.231
InChiKey
NJEKDDOCPZKREE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:13
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.363
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2916399090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— ethyl 3-ethyl-3-phenylpropionate 67478-54-0 C13H18O2 206.285 —— 3-phenylpent-4-enoic acid —— C11H12O2 176.215 3-羟基-3-苯基戊酸 3-hydroxy-3-phenylpentanoic acid 13278-26-7 C11H14O3 194.23 —— (1-phenyl-propyl)-malonic acid 100118-00-1 C12H14O4 222.241 —— 3-phenylpentanoyl chloride 91427-14-4 C11H13ClO 196.677 —— ethyl 3-phenyl-4-pentenoate 60066-61-7 C13H16O2 204.269 3-苯基戊-4-烯醛 3-phenyl-4-pentenal 939-21-9 C11H12O 160.216 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (S)-3-苯基戊酸 (S)-3-phenylvaleric acid 16460-78-9 C11H14O2 178.231 —— (R) 3-phenylvaleric acid —— C11H14O2 178.231 —— (+/-)-methyl 3-phenylpentanoate 30368-22-0 C12H16O2 192.258 —— ethyl 3-ethyl-3-phenylpropionate 67478-54-0 C13H18O2 206.285 —— (R)-ethyl 3-phenylpentanoate 251660-12-5 C13H18O2 206.285 —— (+/-)-3-phenyl-1-pentanol 2845-28-5 C11H16O 164.247 —— 3-phenylpentanoyl chloride 91427-14-4 C11H13ClO 196.677 —— (R)-3-phenylvaleryl chloride 159652-02-5 C11H13ClO 196.677 —— 3-phenyl-pentanoic acid amide 91246-05-8 C11H15NO 177.246 甲基7-苯基壬n酸酯 7-Phenylnonansaeuremethylester 30368-30-0 C16H24O2 248.365 —— 3-phenylpentanamine 89538-62-5 C11H17N 163.263 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Lipase catalyzed acylation of primary alcohols with remotely located stereogenic centres: the resolution of (±)-4,4-dimethyl-3-phenyl-1-pentanol摘要:Enantio selective acylation of some(+/-)-3-alkyl-3-phenyl-1-propanols was performed with enzymes as catalysts. Moderate enantiomeric ratios (E), ranging up to E = 11.6, were obtained. In the resolution, some of the lipases selectively acylated the (+)-enantiomer while others acylated the (-)-enantiomer of the gamma-substituted primary alcohols 1-4. Thus, it is possible to obtain both enantiomers of the alcohols as remaining substrate with high enantiomeric purity. The resolution of (+/-)-4,4-dimethyl-3-phenyl-1-pentanol 4 was extensively studied and screening experiments were conducted to select suitable lipase(s), reaction medium, acyl donor and appropriate temperature combinations to increase the enantiomeric ratio. Chirazyme (R) L-6/chloroform/vinyl propionate/38 degrees C and Chirazyme (R) L-7/di-iso-propyl ether/vinyl propionate/0 degrees C were chosen to obtain both enantiomers, (R)-(+)-4 and (S)-(-)-4, respectively, via sequential resolutions in excellent enantiomeric excess (> 98%) and in 25% and 22% yield, respectively. (c) 2007 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetasy.2007.07.002
-
作为产物:描述:参考文献:名称:Reynolds, American Chemical Journal, 1910, vol. 44, p. 321摘要:DOI:
文献信息
-
Biphenyl derivatives, production thereof and uses as medicines申请人:Boehringer Ingelheim Pharma KG公开号:US06617325B1公开(公告)日:2003-09-09The present invention relates to biphenyl derivatives of general formula wherein Ra to Rg and n are defined as in claim 1, the isomers and salts thereof, particularly the physiologically acceptable salts thereof, which are valuable inhibitors of the microsomal triglyceride-transfer protein (MTP), medicaments containing these compounds and their use, as well as the preparation thereof.
-
Identification of an Esterase Isolated Using Metagenomic Technology which Displays an Unusual Substrate Scope and its Characterisation as an Enantioselective Biocatalyst作者:Declan P. Gavin、Edel J. Murphy、Aoife M. Foley、Ignacio Abreu Castilla、F. Jerry Reen、David F. Woods、Stuart G. Collins、Fergal O'Gara、Anita R. MaguireDOI:10.1002/adsc.201801691日期:2019.6.6Evaluation of an esterase annotated as 26D isolated from a marine metagenomic library is described. Esterase 26D was found to have a unique substrate scope, including synthetic transformations which could not be readily effected in a synthetically useful manner using commercially available enzymes. Esterase 26D was more selective towards substrates which had larger, more sterically demanding substituents
-
Pharmacological characterization of a new series of carbamoylguanidines reveals potent agonism at the H2R and D3R作者:Sabrina Biselli、Merlin Bresinsky、Katharina Tropmann、Lisa Forster、Claudia Honisch、Armin Buschauer、Günther Bernhardt、Steffen PockesDOI:10.1016/j.ejmech.2021.113190日期:2021.3selectivity profile towards the hH2R, e.g. 157 being at least 3800-fold selective within the histamine receptor family. The structural similarities of our monomeric ligands to pramipexole (6), a dopamine receptor agonist, suggested an investigation of the binding behavior at those receptors. The target compounds were (partial) agonists with moderate affinity at the hD2longR and agonists with high affinity即使在今天,组胺H 2受体(H 2 R)在中枢神经系统(CNS)中的作用仍然广为人知。在先前的研究中,许多二聚体,高亲和力和亚型选择性的氨基甲酰基胍型配体,例如UR-NK22(5,p K i = 8.07)被报道为H 2 R激动剂。但是,它们在中枢神经系统中研究H 2 R的适用性因其分子和药代动力学特性(例如高分子量)而受到限制,因此其生物利用度有限。满足对更多类似毒品的H 2的需求R激动剂具有高亲和力,我们合成了一系列含有各种间隔基和侧链部分的单体(硫代)氨基甲酰基胍型配体。这种结构上的简化导致了有效的(部分)激动剂(豚鼠右心房,[ 35 S]GTPγS和β-arrestin2募集试验),其人类(h)H 2 R亲和力在一位纳摩尔范围内(p K i(139,UR-KAT523):8.35; p K i(157,UR-MB-69):8.69)。本文介绍的大多数化合物对hH 2 R表现出出色
-
Norrish type II reactions of acyl azolium salts作者:Andreas Mavroskoufis、Arielle Rieck、Matthew N. HopkinsonDOI:10.1016/j.tet.2021.132497日期:2021.11in N-heterocyclic carbene (NHC) organocatalysis, undergo Norrish type II elimination reactions under irradiation with UVA light in analogy to structurally related aromatic ketones. Moreover, efficient Norrish-Yang cyclization was observed from an adamantyl-substituted derivative. These results further demonstrate the ability of NHCs to influence the absorption properties and photochemical reactivity
-
[EN] PYRIMIDINE COMPOUNDS THAT INHIBIT ANAPLASTIC LYMPHOMA KINASE<br/>[FR] COMPOSÉS PYRIMIDINE QUI INHIBENT LA KINASE DU LYMPHOME ANAPLASIQUE申请人:AMGEN INC公开号:WO2011143033A1公开(公告)日:2011-11-17Compounds of Formula I are useful inhibitors of anaplastic lymphoma kinase. Compounds of Formula I have the following structure: where the definitions of the variables are provided herein.Formula I的化合物是一种有用的间变性淋巴瘤激酶抑制剂。Formula I的化合物具有以下结构:变量的定义在此提供。
表征谱图
-
氢谱1HNMR
-
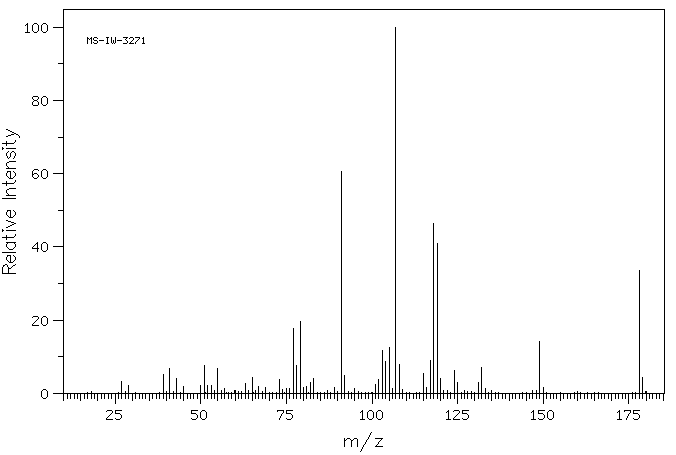
质谱MS
-
碳谱13CNMR
-
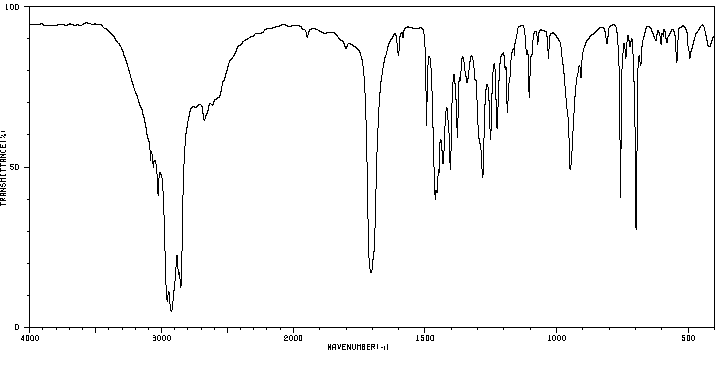
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
限制性核酸内切酶TAQⅠ(TTHHB8I)
阿明洛芬
阿拉洛芬
铁-N-(3-苯基戊二酰)去铁敏B
钙二[(2R)-2-羟基-3-苯丙酸酯]
酮洛芬相关物质C
酪泮酸钠
酪氨酸,3-羟基-b-亚甲基-
苯基丙酮酸缩氨基脲
苯基丁二酸
苯乙酸,a-甲基-4-(4,5,6,7-四氢-2-苯并噻唑基)-
苯丙酸钠盐
苯丙酸钙盐(2:1)
苯丙酸,加合N-环己基并环己胺(1:1)
苯丙酸,b-[[(苯基氨基)羰基]氨基]-
苯丙酸,b-[[(二乙胺基)硫代甲基]硫代]-
苯丙酸,a-[2-[甲基[2-(4-吗啉基)乙基]氨基]-2-羰基乙基]-,(R)-
苯丙酸,a-[(乙酰基硫代)甲基]-,(S)-
苯丙酸,4-羟基-b,2,6-三甲基-,(bR)-
苯丙酸,4-氯-a-(肟基)-
苯丙酸,3-硝基-b-(三氯甲锗烷基)-
苯丙酸,3-氯-a-羟基-
苯丙酸 羟基-4-甲氧基
苄氧羰基-DL-beta-苯丙氨酸
苄基马来酸
苄基丙二酸单酰肼
苄基丙二酸
苄基丁酸
艾司洛尔酸钠
艾司洛尔酸
胆影脒
羧基布洛芬
羟基布洛芬
美索洛芬
米格列奈
米格列奈
碘芬酸
碘番酸
碘泊酸钠
碘泊酸钙
硬脂酰胺丙基鲸蜡硬脂基二甲基铵甲苯磺酸盐
番石榴酸
甲酪氨酸
甲基多巴杂质A
甲基多巴EP杂质B
甲基多巴
甲基3-(4-苄氧基-2-甲基-苯基)丙酸酯
消旋甲酪氨酸
消旋布洛芬赖氨酸盐
消旋卡多曲二元酸杂质