4,6-二叔丁基邻苯三酚 | 3934-77-8
中文名称
4,6-二叔丁基邻苯三酚
中文别名
——
英文名称
4,6-di-tert-butylpyrogallol
英文别名
4,6-di(tert-butyl)-1,2,3-benzenetriol;4,6-di(tert-butyl)pyrogallol;4,6-Di-tert-butyl-pyrogallol;4,6-ditert-butylbenzene-1,2,3-triol
CAS
3934-77-8
化学式
C14H22O3
mdl
MFCD00043703
分子量
238.327
InChiKey
NRVDOWRFIAEMPS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:121 °C(Solv: ligroine (8032-32-4))
-
沸点:348.4±37.0 °C(Predicted)
-
密度:1.092±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.2
-
重原子数:17
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.571
-
拓扑面积:60.7
-
氢给体数:3
-
氢受体数:3
安全信息
-
海关编码:2907299090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Prostaglandin E2 Induces Interaction Between hSlo Potassium Channel and Syk Tyrosine Kinase in Osteosarcoma Cells摘要:前列腺素(PGs)是骨骼对生长因子、激素、炎症或机械应变做出反应的重要介质。在这项研究中,我们发现在 MG63 骨肉瘤细胞中,前列腺素 E2(PGE2)会导致大电导 Ca2+ 依赖性 K+ 通道(BK)开放。这种由 PGE2 介导的通道开放会诱导 BK 的 hSlo α 亚基上各种酪氨酸磷酸化蛋白的招募。由于 hSlo 的 C 端结构域包含一个基于免疫受体酪氨酸的激活基序(ITAM),我们发现据报道主要在造血细胞中表达的 Syk 非受体酪氨酸激酶也在成骨细胞中表达,并在 PGE2 诱导的对接/激活过程后被招募到 ITAM 上。我们的研究表明,Syk/hSlo 的结合依赖于上游 Src 相关酪氨酸激酶的活性,这与免疫受体的经典两步模型相符。最后,我们提供的证据表明,这种 Syk/hSlo 相互作用不会影响骨肉瘤细胞中 BK 通道的电特性。有了这些数据,我们想提出一个新的观点,即 hSlo 通道除了具有传导功能外,还能在骨细胞中作为真正的传导蛋白介入 PGE2 诱导的骨重塑过程。DOI:10.1359/jbmr.2002.17.5.869
-
作为产物:描述:邻苯三酚 生成 4,6-二叔丁基邻苯三酚参考文献:名称:SEBOK, PETER;TIMAR, TIBOR;JASZBERENYL, JOSEPH CSABA;BATTA, GYULA, HETEROCYCLES, 27,(1989) N1, C. 2595-2607摘要:DOI:
文献信息
-
The constitution of the dimers of 4,6-di-t-butyl-3-hydroxy-o-benzoquinone and 5-t-butyl-3-hydroxy-o-benzoquinone作者:N. M. WaldronDOI:10.1039/j39680001914日期:——Evidence is presented which shows that the yellow and white dimers of 4,6-di-t-butyl-3-hydroxy-o-benzoquinone are structures 3,4a,6,8-tetra-t-butyl-4a, 10a-dihydro-9,10a-dihydroxydibenzo-p-dioxin-1,2-dione (II) and 2,3a,5,7-tetra-t-butyl-3a,10a-dihydro-8,10a-dihydroxybenzo[b]cyclopenta[f][1,4]dioxepin-1,10-dione (IV), respectively. The suggested position of substitution of the t-butyl group in 5-t-butylpyrogallol提出的证据表明4,6-二叔丁基-3-羟基-邻苯醌的黄色和白色二聚体是结构3,4a,6,8-四叔丁基-4a,10a-二氢-9,10a-二羟基二苯并-对二恶英-1,2-二酮(II)和2,3a,5,7-四叔丁基-3a,10a-二氢-8,10a-二羟基苯并[ b ]环戊[分别为f ] [1,4] dioxepin-1,10-dione(IV)。已经证实了叔丁基在5-叔丁基邻苯三酚中的取代位置,从而确定了5-叔丁基-3-羟基-邻苯醌的二聚体为5,10-二叔丁基- 2,7-二羟基三环-[5,3,1,1 2,6] dodeca-4,9-二烯-3,8,11,12-四酮(XIII)。在制备5-叔丁基间苯三酚中,使用了新的反应序列以原位连接叔丁基。
-
The oxidation of some pyrogallol and purpurogallin derivatives作者:A. Critchlow、E. Haslam、R.D. Haworth、P.B. Tinker、N.M. WaldronDOI:10.1016/0040-4020(67)85149-4日期:1967.1The oxidation products of a number of 4- and 5-monosubstituted and 4,6-disubstituted pyrogallols are discussed. 4-Alkylpyrogallols yield the corresponding 4′,7-dialkylpurpurogallins and the structures of the products resulting from the further oxidation of these compounds with alkaline hydrogen peroxide are elucidated. Oxidation of 5-mono and 4,6-dialkylpyrogallol derivatives yield characteristic white
-
Synthesis, chemical properties, and crystal structure of 2,4,6,8-tetra(tert-butyl)-9-hydroxyphenoxazin-1-one作者:V. I. Simakov、Yu. Yu. Gorbanev、T. E. Ivakhnenko、V. G. Zaletov、K. A. Lyssenko、Z. A. Starikova、E. P. Ivakhnenko、V. I. MinkinDOI:10.1007/s11172-009-0182-4日期:2009.7The reaction of di(tert-butyl) derivatives of pyrocatechol with 2,6-dihydroxyaniline afforded 2,4,6,8-tetra(tert-butyl)-9-hydroxyphenoxazin-1-one. The chemical properties of the reaction product and its ability to form complexes with metal salts as the tridentate ligand were investigated. The structure of hydroxyphenoxazinone was established by X-ray diffraction.
-
Structures of oxidation products of 4,6-di-tert-butylpyrogallol作者:A. I. Shif、S. N. Lyubchenko、O. Ya. Borbulevych、O. V. Shishkin、K. A. Lyssenko、L. P. OlekhnovichDOI:10.1007/bf02494416日期:1999.1Oxidation of 4,6-di-tert-butylpyrogallol gave two dimeric products instead of the expected 4,6-di-tert-butyl-3-hydroxy-1,2-benzoquinone (2). It was established by X-ray diffraction analysis that the first product has the structure of tetra-tert-butyl-6, 10a-dihydroxy-1,2-dioxo-3,4a,7,9-1,2,4a, 10a-tetrahydrodibenzo-1,4-dioxine. From this it follows that compound 2 undergoes regio- and stereospecific4,6-二叔丁基焦酚的氧化得到两种二聚产物,而不是预期的 4,6-二叔丁基-3-羟基-1,2-苯醌 (2)。X-射线衍射分析确定第一产物具有四叔丁基-6, 10a-二羟基-1,2-二氧-3,4a,7,9-1,2,4a, 10a的结构-四氢二苯并-1,4-二恶英。由此可知,化合物 2 根据 [2π+4π]-环加成机制,即杂 Diels-Alder 反应进行区域和立体定向二聚化。1 H NMR 光谱中信号的双强度表明第二个产物 2,6,4', 6'-四叔丁基-4,4'-二羟基-3,5,3' 的对称结构,5'-tetraoxo-4,4'-bi(cyclohexene),它是在连苯三酚氧化的中间体(即,其)重组 (r+r orl+l) 后形成的对映异构体的外消旋物,
-
GELS COMPRISING A HYDROPHOBIC MATERIAL申请人:Procter & Gamble International Operations SA.公开号:EP3431143A1公开(公告)日:2019-01-23The gel compositions as described herein, are less affected by changes in humidity, are readily formed into complex shapes, and can contain high loadings of the hydrophobic material, even when the material is not highly hydrophobic, and provide a more controlled release of the hydrophobic material.
表征谱图
-
氢谱1HNMR
-
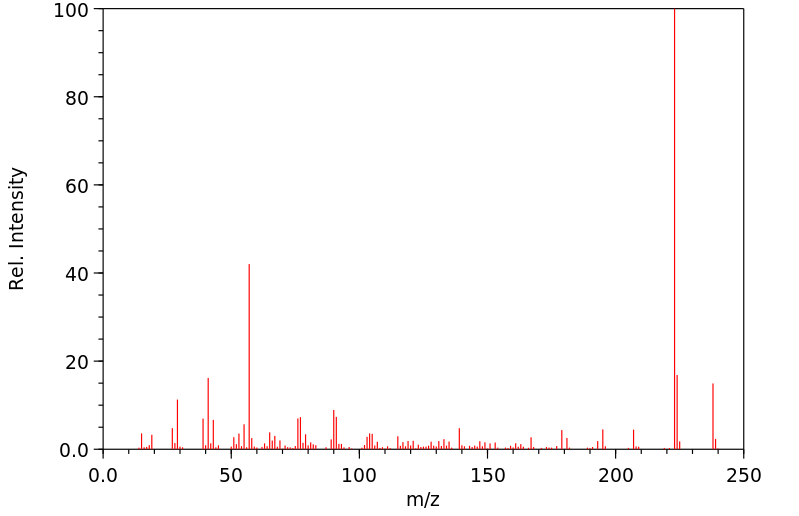
质谱MS
-
碳谱13CNMR
-
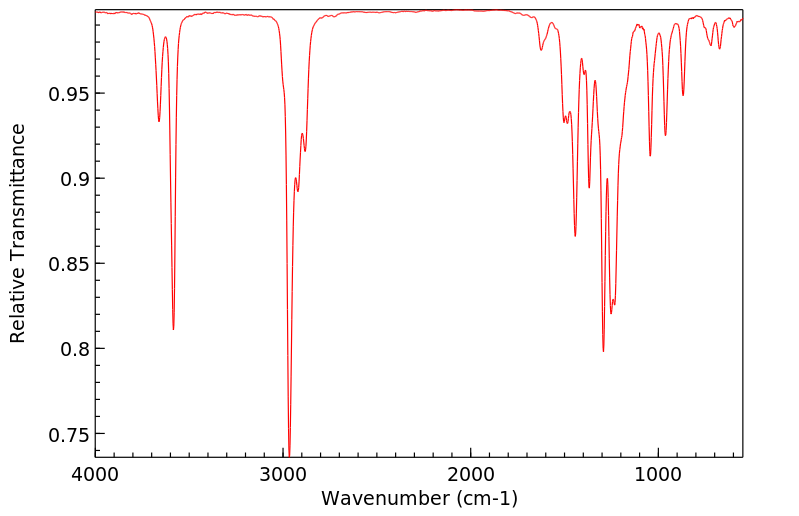
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚