4-吲哚-3-硝基酚 | 65370-06-1
中文名称
4-吲哚-3-硝基酚
中文别名
2-乙基硫代甲基酚
英文名称
2-(ethylthiomethyl)-phenol
英文别名
2-[(Ethylsulfanyl)methyl]phenol;2-(ethylsulfanylmethyl)phenol
CAS
65370-06-1
化学式
C9H12OS
mdl
MFCD01632292
分子量
168.26
InChiKey
QZBBPVLBIUUYRH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
保留指数:1404
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:11
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:45.5
-
氢给体数:1
-
氢受体数:2
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:CABRAS, PAOLO;MELONI, MARCO;PLUMITALLO, ANTONIO;GENNARI, MARA, J. CHROMATOGR., 462,(1989) C. 430-434摘要:DOI:
-
作为产物:描述:参考文献:名称:乙硫芬威的碱性水解:动力学研究和机理降解摘要:本文研究了碱溶液中乙硫基苯并[2-乙基硫代甲基(苯基)-N-甲基氨基甲酸酯]的水解。已经使用分光光度法和液相色谱技术研究了反应动力学。速率常数是根据拟议的一阶动力学模型确定的。正活化熵ΔS ≠ = +100.07 J mol -1 K -1,并且不存在一般的碱性催化作用,表明存在E1cB水解机理,涉及异氰酸甲酯的形成。下列事实证实了这一结果:乙硫芬威很适合Brönsted和Hammett系,这是从一系列取代的N获得的氨基甲酸甲酯,其在水性介质中的分解遵循E1cB机制。©2012 Wiley Periodicals,Inc.国际化学杂志Kinet 45:118–124,2013DOI:10.1002/kin.20748
-
作为试剂:描述:水杨醇 、 乙硫醇 在 4-吲哚-3-硝基酚 作用下, 以 水 为溶剂, 25.0 ℃ 、26.66 kPa 条件下, 以128 g of 2-ethylthiomethyl-phenol (ca. 95% of theory) were obtained的产率得到4-吲哚-3-硝基酚参考文献:名称:Process for the preparation of alkylthiomethylphenols and摘要:本发明提供了一种制备通式为##STR1##的烷基硫代甲基酚或芳基硫代甲基酚的方法,其中m、n、R和R.sup.1的含义如规范中所述,其中将通式为##STR2##的羟甲基酚与巯基或硫酚在20℃到200℃的温度下反应,如有必要,在稀释剂的存在下。公开号:US04358616A1
文献信息
-
Preparation of alkylthiomethylphenols申请人:Bayer Aktiengesellschaft公开号:US04091037A1公开(公告)日:1978-05-23A process for the preparation of an alkylthiomethylphenol of the formula ##STR1## COMPRISING REACTING A DIALKYLAMINOMETHYLPHENOL OF THE FORMULA ##STR2## WITH A THIOCARBOXYLIC ACID S-ester of the formula ##STR3## in which R.sup.1 is optionally substituted alkyl with 1 to 12 carbon atoms or optionally substituted phenyl, R.sup.2 and R.sup.3 each independently is hydrogen, alkyl with 1 to 5 carbon atoms, halogen or nitro, or together form a benzene ring or cycloalkane ring with 3 to 5 carbon atoms which is fused to the phenyl ring, R.sup.4 and R.sup.5 each independently is alkyl with 1 to 6 carbon atoms, or together with the nitrogen atom form a five- or six-membered heterocyclic ring, R.sup.6 is hydrogen or alkyl with 1 to 6 carbon atoms, and n is 1, 2 or 3. The process may be carried out in a solvent, advantageously at a temperature from about 100.degree. to 150.degree. C, at about normal pressure using phenol as a catalyst with about 0.9 to 1.5 moles of thiocarboxylic acid S-ester per mole of dialkylaminomethylphenol.一种制备式的烷基硫代甲基酚的方法,包括将式的二烷基氨基甲基酚与式的硫代羧酸S-酯反应,其中R.sup.1是可选的取代烷基,碳原子数为1到12,或可选的取代苯基,R.sup.2和R.sup.3各自独立地是氢、碳原子数为1到5的烷基、卤素或硝基,或者一起形成与苯环相融合的具有3到5个碳原子的苯环或环戊烷环,R.sup.4和R.sup.5各自独立地是碳原子数为1到6的烷基,或者与氮原子一起形成五元或六元杂环,R.sup.6是氢或碳原子数为1到6的烷基,n为1、2或3。该方法可以在溶剂中进行,优选在约100°C到150°C的温度下,在约正常压力下使用苯酚作为催化剂,每摩尔二烷基氨基甲基酚使用约0.9到1.5摩尔硫代羧酸S-酯。
-
Iodine-Mediated Direct Generation of <i>o</i> -Quinone Methides at Room Temperature: A Facile Protocol for the Synthesis of <i>ortho</i> -Hydroxybenzyl Thioethers作者:R. Sidick Basha、Chia-Wei Chen、Daggula Mallikarjuna Reddy、Chin-Fa LeeDOI:10.1002/asia.201800233日期:2018.9.4An iodine‐mediated preparation of ortho‐quinone methides (o‐QMs) from ortho‐hydroxybenzyl alcohols by a C−O bond scission strategy is described. The in situ generated o‐QMs were then employed for C−S bond formation by thio‐Michael addition of thiols to provide the ortho‐hydroxybenzyl thioethers (o‐HBT) in moderate to excellent yields.
-
In situ fluorescent labeling of highly volatile methylamine with 8-(4,6-dichloro-1,3,5-triazinoxy)quinoline作者:Huimin Ma、Ute Jarzak、Wolfram ThiemannDOI:10.1039/b101711j日期:——The first in situ fluorescent labeling probe for monitoring of highly volatile methylamine, 8-(4,6-dichloro-1,3,5-triazinoxy)quinoline, has been designed, synthesized and evaluated. The probe labels spectroscopically inert methylamine, causing a large change in fluorescence properties through suppression of the internal charge transfer, and thus can serve as an in situ labeling probe. This applicability has been demonstrated by measuring methylamine released during hydrolysis of N-methylcarbamates such as ethiofencarb.
-
N,N'-unsymmetrisch substituierte Thio-bis-amine, Verfahren zu ihrer Herstellung und ihre Verwendung als Insektizide, Akarizide und Nematozide申请人:BAYER AG公开号:EP0005780A1公开(公告)日:1979-12-12Die vorliegende Erfindung betrifft N,N'-unsymmetrisch substituierte Thio-bis-amine der allgemeinen Formel I in welcher A2, R, R1, R2in die in der Beschreibung angegebene Bedeutung besitzen. Man erhält sie, wenn man a) Oxime der Formel mit sulfenylierten Carbamoylhalogeniden der Formel umsetzt, oder b) gegebenenfalls die nach dem Verfahren (a) erhältlichen sulfenylierten Oximcarbamate der Formel mit Verbindungen der Formel umsetzt. Die erfindungsgemäßen Verbindungen zeigen insektizide, akarizide und nematozide Wirkung.
-
Verfahren zur Herstellung von Alkyl- bzw. Arylthiomethyl-phenolen申请人:BAYER AG公开号:EP0011091A1公开(公告)日:1980-05-28Die vorliegende Erfindung betrifft ein neues Verfahren zur Herstellung von Alkyl- bzw. Arylthiomethylphenolen der Formel I das dadurch gekennzeichnet ist, daß man Hydrooxymethylphenole der Formel II mit Mercaptanen oder Thiophenolen bei Temperaturen von 20 bis 200 , gegebenenfalls in Anwesenheit eines Verdünnungsmittels umsetzt.
表征谱图
-
氢谱1HNMR
-
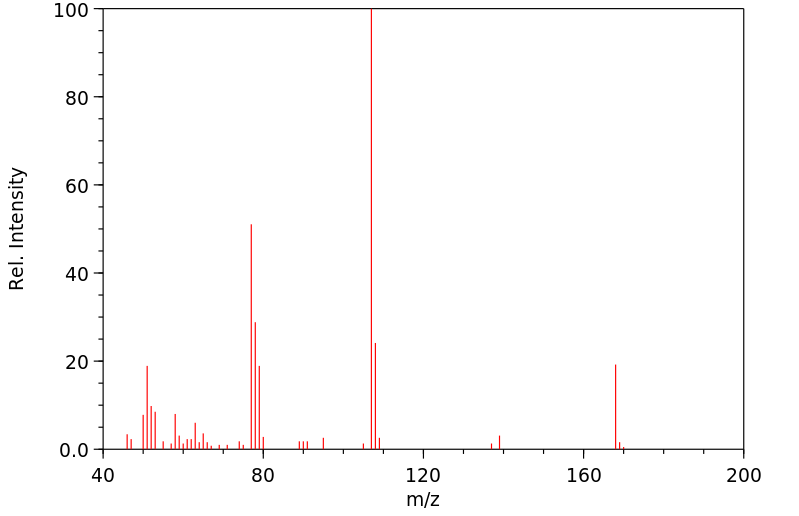
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚