ethyl (E)-2-cyano-3-(naphthalen-2-yl)acrylate
中文名称
——
中文别名
——
英文名称
ethyl (E)-2-cyano-3-(naphthalen-2-yl)acrylate
英文别名
Ethyl 2-cyano-trans-3-(2-naphthyl)acrylate;ethyl (E)-2-cyano-3-naphthalen-2-ylprop-2-enoate
CAS
——
化学式
C16H13NO2
mdl
——
分子量
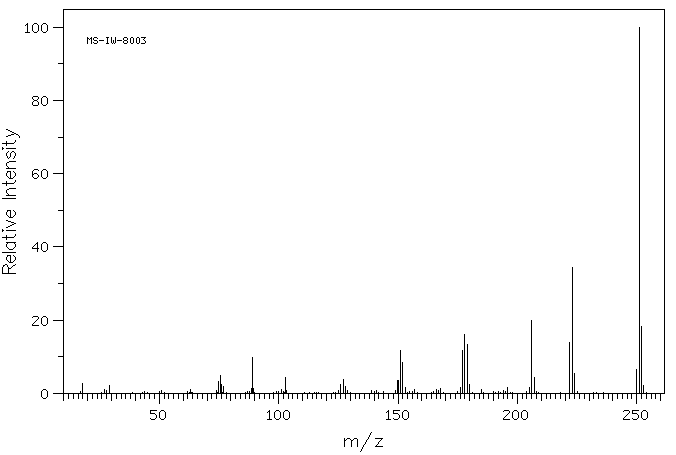
251.285
InChiKey
OCPOYALXTVXRED-XNTDXEJSSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:19
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.12
-
拓扑面积:50.1
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为反应物:描述:ethyl (E)-2-cyano-3-(naphthalen-2-yl)acrylate 在 氨 作用下, 以 甲醇 、 水 为溶剂, 反应 0.5h, 以35%的产率得到4,6-bis(4-naphthyl)-3,5-dicyano-5-ethoxycarbonyl-2-piperidinone参考文献:名称:One-Pot Formation of Functionalised 2-Piperidinones from Arylidenecyanoacetates and Methanolic Ammonia via Tandem Reactions摘要:Aryl aldehydes react with ethyl cyanoacetate in methanolic ammonium acetate to expeditiously furnish, besides high yields of arylidenecyanoacetates, 4,6-diaryt-3,5-dicyano-5-ethoxycarbonyl-2-piperidinones in low yields. But preformed arylidenecyanoacetates react with methanolic ammonia to furnish the same functionalised 2-piperidinones in much better yields. The actual stereostructure of one of the products was determined by single crystal X-ray diffraction analysis, and novel tandem reactions occurring in one pot are proposed for the formation of the products.DOI:10.3987/com-07-s(u)59
-
作为产物:描述:2-萘甲醛 、 氰乙酸乙酯 在 xonotlite 、 potassium tert-butylate 作用下, 反应 1.0h, 以89%的产率得到ethyl (E)-2-cyano-3-(naphthalen-2-yl)acrylate参考文献:名称:Knoevenagel缩合反应的催化摘要:单独的硬硅钙石或通过叔丁醇钾的掺杂使碱性硬硅钙石更为碱性,可催化芳族醛与丙二腈或氰基乙酸烷基酯之间的标题反应。在环境温度下,此程序可特别提供高收率的E烯烃Knoevenagel产品。DOI:10.1016/s0040-4039(00)88928-5
文献信息
-
Critical assessment of the efficiency of chitosan biohydrogel beads as recyclable and heterogeneous organocatalyst for C–C bond formation作者:Dennis Kühbeck、G. Saidulu、K. Rajender Reddy、David Díaz DíazDOI:10.1039/c1gc15925a日期:——The effectiveness of neutral pH chitosan hydrogel beads (CSHB) as a green organocatalyst for a variety of CâC bond forming reactions (i.e. aldol reaction, Knoevenagel condensation, nitroaldol (Henry) reaction, Michael addition) has been comprehensively evaluated. Reaction rates, conversions and selectivities were studied as a function of a series of input variables including size, pH and reactive surface area of the beads, catalyst loading, temperature, molecular weight of the biopolymer, concentration, solvent system and molar ratio of reactants. Moreover, the catalytic biohydrogel beads were characterized by a variety of techniques including, among others, SEM, FT-IR, TGA and DSC.
-
Development of the First Two-Pore Domain Potassium Channel TWIK-Related K<sup>+</sup> Channel 1-Selective Agonist Possessing in Vivo Antinociceptive Activity作者:Delphine Vivier、Ismail Ben Soussia、Nuno Rodrigues、Stéphane Lolignier、Maïly Devilliers、Franck C. Chatelain、Laetitia Prival、Eric Chapuy、Geoffrey Bourdier、Khalil Bennis、Florian Lesage、Alain Eschalier、Jérôme Busserolles、Sylvie DuckiDOI:10.1021/acs.jmedchem.6b01285日期:2017.2.9development of a novel class of analgesic drugs, suggesting that activation of TREK-1 could result in pain inhibition. Here, we report the synthesis of a series of substituted acrylic acids (1–54) based on our previous work with caffeate esters. The analogues were evaluated for their ability to modulate TREK-1 channel by electrophysiology and for their in vivo antinociceptive activity (acetic acid-induced
-
A rhodium-catalysed conjugate addition/cyclization cascade for the asymmetric synthesis of 2-amino-4<i>H</i>-chromenes作者:Zhiqian Chang、Huilong Zhu、Changhui Wu、Junhao Xing、Xiaowei DouDOI:10.1039/d0ob02404j日期:——enantioselective synthesis of 2-amino-4H-chromenes via the cascade rhodium-catalysed conjugate addition/hetero Thorpe–Ziegler reaction is reported. Moderate to good yields (up to 98%) and high enantioselectivities (up to 92% ee) were obtained with a chiral diene-coordinated rhodium complex as the catalyst. This protocol remedies the methodological deficiency in the asymmetric synthesis of 4-aryl 2-amino-4H-chromenes
-
Multiple Absolute Stereocontrol in Pd-Catalyzed [3+2] Cycloaddition of Oxazolidinones and Trisubstituted Alkenes Using Chiral Ammonium–Phosphine Hybrid Ligands作者:Naomichi Imagawa、Yuya Nagato、Kohsuke Ohmatsu、Takashi OoiDOI:10.1246/bcsj.20160053日期:2016.6.15The development of a Pd-catalyzed highly enantio- and diastereoselective [3+2] cycloaddition of 5-vinyloxazolidinones and activated trisubstituted alkenes is described in detail. This protocol for ...
-
Catalyst-Free [3 + 3] Annulation/Oxidation of Cyclic Amidines with Activated Olefins: When the Substrate Olefin Is Also an Oxidant作者:Wendan Han、Yuanhang Li、Kaki Raveendra Babu、Jing Li、Yuhai Tang、Yong Wu、Silong XuDOI:10.1021/acs.joc.1c00717日期:2021.6.4DBN (1,5-diazabicyclo(4.3.0)non-5-ene) with activated olefins, i.e., 2-arylidenemalononitriles and 2-cyano-3-aryl acrylates, to afford tricyclic 2-pyridones and pyridin-2(1H)-imines, respectively. The mechanism has been proposed based on DFT calculations. In the reaction, the cyclic amidines serve as C,N-bisnucleophiles for the cyclization, while the olefins play a dual role by acting as both reactants
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
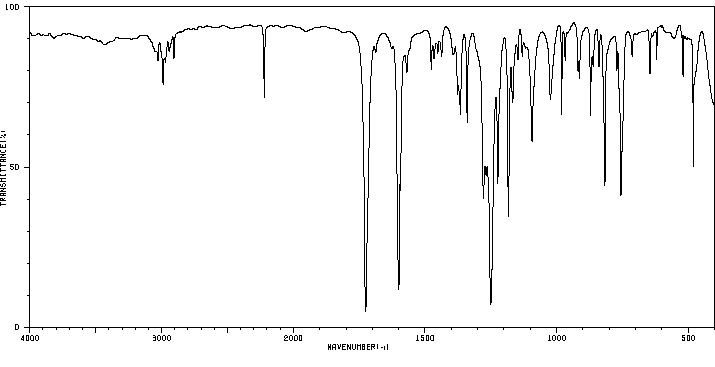
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮