4-(Ethoxycarbonyl)-4'-methylazobenzene | 74763-69-2
中文名称
——
中文别名
——
英文名称
4-(Ethoxycarbonyl)-4'-methylazobenzene
英文别名
4-p-tolylazo-benzoic acid ethyl ester;4-Aethoxycarbonyl-4'-methyl-azobenzol;Ethyl 4'-methylazobenzene-4-crboxylate;ethyl 4-[(4-methylphenyl)diazenyl]benzoate
CAS
74763-69-2
化学式
C16H16N2O2
mdl
——
分子量
268.315
InChiKey
ZUWQMBVFXIWLQS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:410.0±28.0 °C(Predicted)
-
密度:1.09±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):5.1
-
重原子数:20
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.19
-
拓扑面积:51
-
氢给体数:0
-
氢受体数:4
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯佐卡因 p-aminoethylbenzoate 94-09-7 C9H11NO2 165.192
反应信息
-
作为反应物:描述:4-(Ethoxycarbonyl)-4'-methylazobenzene 在 锌 作用下, 以 吡啶 、 溶剂黄146 为溶剂, 生成 4-(Ethoxycarbonyl)-4'-methylhydrazobenzene参考文献:名称:Photobenzidine rearrangements. 6. Mechanism of the photodecomposition of 1,4-diaryl-1,4-dialkyl-2-tetrazenes摘要:DOI:10.1021/jo01310a036
-
作为产物:参考文献:名称:通过苯胺的氧化脱氢偶联反应苯碘 (III) 二乙酸酯 (PIDA) 介导合成芳香偶氮化合物:范围和机制摘要:已经开发了一种高效且环境友好的方法,用于通过二乙酸苯碘 (III) (PIDA) 介导的苯胺氧化脱氢偶联以高产率合成对称和不对称的芳香偶氮化合物。该反应的范围对于均二聚和交叉二聚都很宽泛。基于结构特征的关键中间体提出了一种合理的反应机制,表明乙醇参与了反应途径。快速反应、无金属条件和官能团耐受性是这种方法的优点。DOI:10.1002/ejoc.201301209
文献信息
-
Highly efficient synthesis of azos catalyzed by the common metal copper (0) through oxidative coupling reactions作者:Jiaqing Wang、Jing He、Cong Zhi、Bin Luo、Xinming Li、Yue Pan、Xueqin Cao、Hongwei GuDOI:10.1039/c4ra00749b日期:——bridged aromatic azo compounds (AAzos) from aromatic amines was developed by using red copper as catalyst. Despite numerous efforts towards the catalytic synthesis of symmetric and asymmetric AAzos derivatives, most reactions present certain drawbacks inhibiting their industrial applications, such as laborious multi-step processes, harsh reaction conditions and expensive reagents. And the synthesis
-
Synthesis of (2<i>H</i>)-Indazoles and Dihydrocinnolinones through Annulation of Azobenzenes with Vinylene Carbonate under Rh(III) Catalysis作者:Min Seo Park、Kyeongwon Moon、Harin Oh、Ji Yoon Lee、Prithwish Ghosh、Ju Young Kang、Jung Su Park、Neeraj Kumar Mishra、In Su KimDOI:10.1021/acs.orglett.1c01866日期:2021.7.16
表征谱图
-
氢谱1HNMR
-
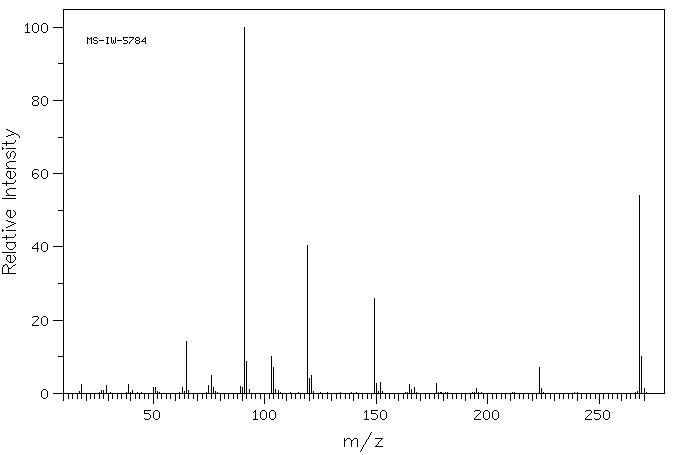
质谱MS
-
碳谱13CNMR
-
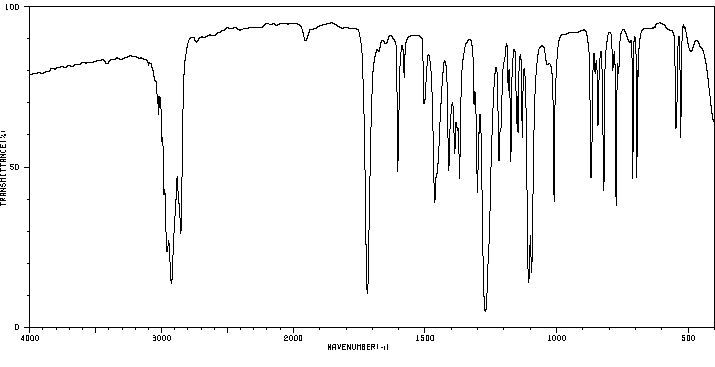
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黑洞猝灭剂-2,BHQ-2ACID
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S,24S)-
颜料橙61
阿利新黄GXS
阳离子红X-GTL
阳离子红5BL
阳离子橙RN
阳离子橙GLH
间甲基红
镨(3+)丙烯酰酸酯
镍酸酯(1-),[3-羟基-4-[(4-甲基-3-硫代苯基)偶氮]-2-萘羧酸根(3-)]-,氢
锂3-({4-[(4-羟基苯基)偶氮]-5-甲氧基-2-甲基苯基}偶氮)苯磺酸酯
钴,[二[m-[[1,2-二苯基-1,2-乙二酮1,2-二(肟酸根-kO)](2-)]]四氟二硼酸根(2-)-kN1,kN1',k2,kN2']-,(SP-4-1)-
钠5-氯-2-羟基-3-[(2-羟基-4-{[(4-甲基苯基)磺酰基]氧基}苯基)偶氮]苯磺酸酯
钠5-[[3-[[5-[[4-[[[4-[(4,5-二氢-3-甲基-5-氧代-1H-吡唑-4-基)偶氮]苯基]氨基]羰基]苯基]偶氮]-2,4-二羟基苯基]偶氮]-4-羟基苯基]偶氮]水杨酸盐
钠4-[(4-氨基苯基)偶氮]苯甲酸酯
钠4-[(4-{[4-(二乙基氨基)苯基]偶氮}苯基)偶氮]苯磺酸酯
钠4-[(4-{[2-羟基-5-(2-甲基-2-丙基)苯基]偶氮}苯基)偶氮]苯磺酸酯
钠4-({3-甲氧基-4-[(4-甲氧基苯基)偶氮]苯基}偶氮)苯磺酸酯
钠3-[4-(2-羟基-5-甲基-苯基)偶氮苯基]偶氮苯磺酸酯
钠3-({5-甲氧基-4-[(4-甲氧基苯基)偶氮]-2-甲基苯基}偶氮)苯磺酸酯
钠3-({4-[(4-羟基-2-甲基苯基)偶氮]-3-甲氧基苯基}偶氮)苯磺酸酯
金莲橙O
重氮基烯,苯基[4-(三氟甲基)苯基]-
重氮基烯,二[4-(1-甲基乙基)苯基]-,(Z)-
重氮基烯,二[4-(1-甲基乙基)苯基]-,(E)-
重氮基烯,[4-[(2-乙基己基)氧代]-2,5-二甲基苯基](4-硝基苯基)-
重氮基烯,1,2-二(4-丙氧基苯基)-,(1E)-
重氮基烯,(2-氯苯基)苯基-
酸性金黄G
酸性棕S-BL
酸性媒染棕
酸性媒介棕6
酸性媒介棕48
酸性媒介棕4
酸性媒介棕24
邻氨基偶氮甲苯
达布氨乙基甲硫基磺酸盐
赛甲氧星
茴香酸盐己基
茜素黄 R 钠盐
苯重氮化,2-甲氧基-5-甲基-4-[(4-甲基-2-硝基苯基)偶氮]-,氯化
苯酰胺,4-[4-(2,3-二氢-1,4-苯并二噁英-6-基)-5-(2-吡啶基)-1H-咪唑-2-基]-
苯酚,4-(1,1-二甲基乙基)-2-(苯偶氮基)-
苯酚,2-甲氧基-4-[(4-硝基苯基)偶氮]-
苯胺棕
苯胺,4-[(4-氯-2-硝基苯基)偶氮]-
苯磺酸,3,3-6-(4-吗啉基)-1,3,5-三嗪-2,4-二基二亚氨基2-(乙酰基氨基)-4,1-亚苯基偶氮二-,盐二钠
苯磺酸,2-[(4-氨基-2-羟基苯基)偶氮]-
苯甲酸,5-[[4-[(乙酰基氨基)磺酰]苯基]偶氮]-2-[[3-(三氟甲基)苯基]氨基]-(9CI)