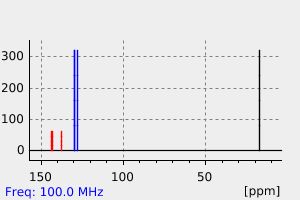
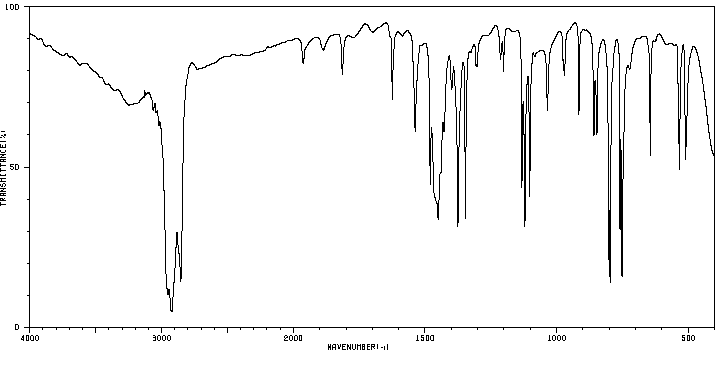
1,6-dimethylphenazine | 58718-43-7
中文名称
——
中文别名
——
英文名称
1,6-dimethylphenazine
英文别名
——
CAS
58718-43-7
化学式
C14H12N2
mdl
——
分子量
208.263
InChiKey
XEWNMXMURNDKRC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:16
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:25.8
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:描述:参考文献:名称:Kidani; Otomasu, Pharmaceutical Bulletin, 1956, vol. 4, p. 391,394摘要:DOI:
-
作为产物:描述:参考文献:名称:Malaviya; Dutt, 1934, vol. 4, p. 319,321摘要:DOI:
文献信息
-
Low-temperature Photolysis of<i>ortho</i>-Substituted Azidobiphenyls. Formation of Phenazines from 2,2′-Diazidobiphenyls作者:Akira YabeDOI:10.1246/bcsj.53.2933日期:1980.10in a rigid matrix. The phenazine formation results from the 1,1′-C–C bond fission of the biphenyl nucleus and suggests the aziridine intermediates. The low-temperature photolysis of 2-azido-2′-methylbiphenyl and 2-azido-2′,4′,6′-trimethylbiphenyl leads to the triplet-derived phenanthridine derivatives as major products.
-
Phenazine derivatives as antimicrobial agents申请人:University of Florida Research Foundation, Incorporated公开号:US11053205B2公开(公告)日:2021-07-06The present invention provides novel phenazine derivatives, such as compounds of Formula (I) (e.g., Formulae (II)-(XIX)), and pharmaceutically acceptable salts thereof. The compounds of the invention are expected to be antimicrobial agents and may act by a microbial warfare strategy (e.g., a reactive oxygen species (ROS)-based competition strategy). The present invention also provides pharmaceutical compositions, kits, uses, and methods that involve the compounds of the invention and may be useful in preventing or treating a microbial infection (e.g., a bacterial infection or mycobacterial infection) in a subject, inhibiting the growth and/or reproduction of a microorganism (e.g., a bacterium or mycobacterium), killing a microorganism (e.g., a bacterium or mycobacterium), inhibiting the formation and/or growth of a biofilm, reducing or clearing a biofilm, and/or disinfecting a surface.
-
YABE AKIRA, BULL. CHEM. SOC. JAP., 1980, 53, NO 10, 2933-2937作者:YABE AKIRADOI:——日期:——
-
[EN] PHENAZINE DERIVATIVES AS ANTIMICROBIAL AGENTS<br/>[FR] DÉRIVÉS DE PHÉNAZINE UTILISÉS COMME AGENTS ANTIMICROBIENS申请人:UNIV OF RESEARCH FOUNDATION INCORPORATED公开号:WO2018152436A1公开(公告)日:2018-08-23The present invention provides novel phenazine derivatives, such as compounds of Formula (I) (e.g., Formulae (II) - (XIX)), and pharmaceutically acceptable salts thereof. The compounds of the invention are expected to be antimicrobial agents and may act by a microbial warfare strategy (e.g., a reactive oxygen species (ROS)-based competition strategy). The present invention also provides pharmaceutical compositions, kits, uses, and methods that involve the compounds of the invention and may be useful in preventing or treating a microbial infection (e.g., a bacterial infection or mycobacterial infection) in a subject, inhibiting the growth and/or reproduction of a microorganism (e.g., a bacterium or mycobacterium), killing a microorganism (e.g., a bacterium or mycobacterium), inhibiting the formation and/or growth of a biofilm, reducing or clearing a biofilm, and/or disinfecting a surface.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮