Benzylidene-2-naphthylamine | 75472-73-0
中文名称
——
中文别名
——
英文名称
Benzylidene-2-naphthylamine
英文别名
(E)-N-(naphthalen-2-yl)-1-phenylmethanimine;(E)-N-(2-naphthyl)-1-phenylmethanimine
CAS
75472-73-0
化学式
C17H13N
mdl
——
分子量
231.297
InChiKey
CKIGNOCMDJFFES-QGOAFFKASA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:100-101 °C
-
沸点:400.5±14.0 °C(Predicted)
-
密度:1.02±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.59
-
重原子数:18.0
-
可旋转键数:2.0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:12.36
-
氢给体数:0.0
-
氢受体数:1.0
反应信息
-
作为反应物:描述:参考文献:名称:Kozlov; Sauts; Gusak, Russian Journal of Organic Chemistry, 2000, vol. 36, # 4, p. 531 - 538摘要:DOI:
-
作为产物:参考文献:名称:Solvent-free synthesis of azomethines, spectral correlations and antimicrobial activities of some E-benzylidene-4-chlorobenzenamines摘要:一些偶氮甲碱,包括取代苄叉-4-氯苯胺(E-亚胺),已在无溶剂条件下通过飞灰:PTS催化的微波辅助缩合4-氯苯胺和取代苯甲醛合成。亚胺的产率已发现超过85%。所有亚胺的纯度已通过其物理常数以及紫外、红外和核磁共振光谱数据进行检查。这些光谱数据已通过单一和多元线性回归分析与Hammett取代常数以及F和R参数相关联。从统计分析的结果中,研究了取代基对上述光谱数据的影响。所有亚胺的抗菌活性已采用标准方法进行研究。DOI:10.4314/bcse.v29i2.10
文献信息
-
Cooperative catalysis of molybdenum with organocatalysts for distribution of products between amines and imines作者:Di Wu、Qingqing Bu、Cheng Guo、Bin Dai、Ning LiuDOI:10.1016/j.mcat.2021.111415日期:2021.3Multi-amino groups and nitrogen donors compound was discovered as an organocatalyst for N-alkylation of alcohols with amines in the presence of Mo(CO)6. The Mo(CO)6/organocatalyst binary system has shown to be a highly active catalyst for the N-alkylation reaction between alcohols and amines with excellent tolerance of variable starting materials bearing different functional groups. Of particular note
-
Process for production of amines申请人:Terada Masahiro公开号:US20070142639A1公开(公告)日:2007-06-21A process for producing an amine which is characterized by reacting an imine with a nucleophilic compound (except a trialkylsilyl vinyl ether) in the presence of a phosphoric acid derivative represented by the formula (1): wherein A 1 represents a spacer; X 1 and X 2 represent each independently a divalent nonmetal atom or a divalent nonmetal atomic group; and Y 1 is oxygen or sulfur. The invention provides a process by which amines (particularly optically active amines) useful as intermediates of medicines, agricultural chemicals, or the like can be produced without special post-treatment in high yield at high optical purity; and phosphoric acid derivatives (particularly optically active phosphoric acid derivatives) useful in the production of the amines.
-
A Convenient Synthesis of Aminoacids by ZnBr<sub>2</sub> Promoted Reaction of Ketene <i>bis</i>(Trimethylsilyl) Acetals with Aldimines作者:M. Mladenova、M. BellassouedDOI:10.1080/00397919308009832日期:1993.3Abstract The ZnBr2 promoted addition of ketene bis(trimethylsilyl) acetals to aromatic aldimines affords β-arylaminoacids in good to excellent yields. Under the same reaction conditions vinylic ketene bis(trimethylsilyl) acetals give exclusively or mainly δ - phenylaminoacids.
-
Synthesis of benzo[f]quinoline derivatives by condensation of N-arylmethylene-2-naphthylamines with acetylcyclohexane and 1-acetylcyclohexene作者:V. I. GrachekDOI:10.1007/s11176-005-0094-4日期:2004.11N -Arylmethylene-2-naphthylamines react with acetylcyclohexane and 1-acetylcyclohexene under mild conditions to afford 2-aryl-2-(2-naphthylamino)ethyl cyclohexyl and 1-cyclohexenyl ketones, respectively. Under more severe conditions (110°C), the reaction is accompanied by cyclization with formation of 3-aryl-1-cyclohexyl(or 1-cyclohexenyl)benzo[ f ]quinolines. Proper choice of the amount of catalyst
-
Role of imine isomerization in the stereocontrol of the Staudinger reaction between ketenes and imines作者:Fernando P. Cossío、Abel de Cózar、Miguel A. Sierra、Luis Casarrubios、Jaime G. Muntaner、Bimal K. Banik、Debasish BandyopadhyayDOI:10.1039/d1ra06114c日期:——allowed determining that the stereochemistry of the Staudinger reaction between ketenes and imines is strongly associated with the nature of the imine, which affects the two steps of the reaction. The first step, namely the nucleophilic attack of the sp2-hybridized nitrogen atom of the imine on the sp-hybridized carbon atom of the ketene, is affected by the energetically accessible in situ isomerization
表征谱图
-
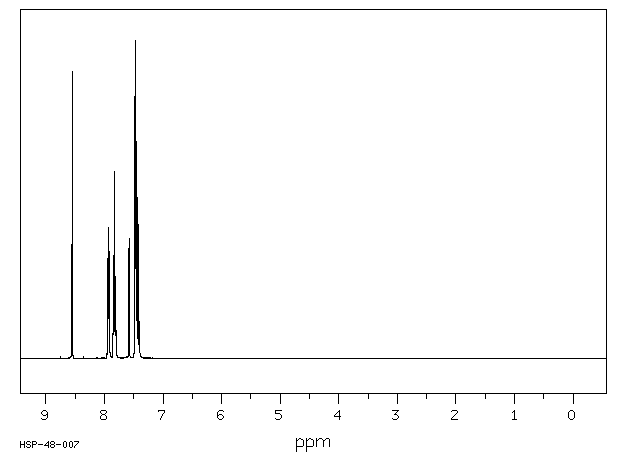
氢谱1HNMR
-
质谱MS
-
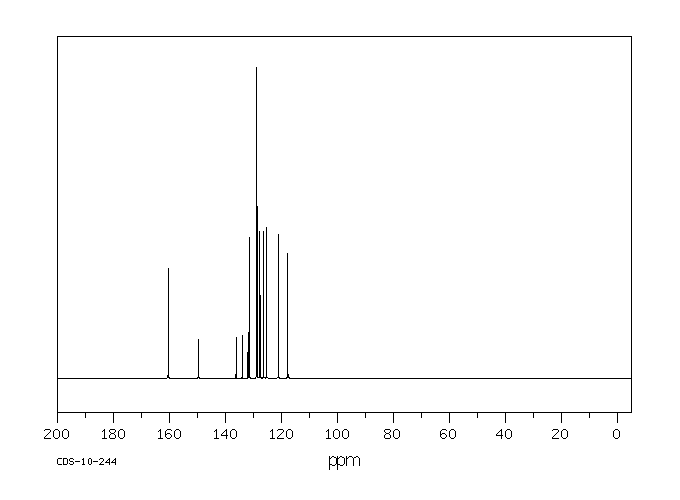
碳谱13CNMR
-
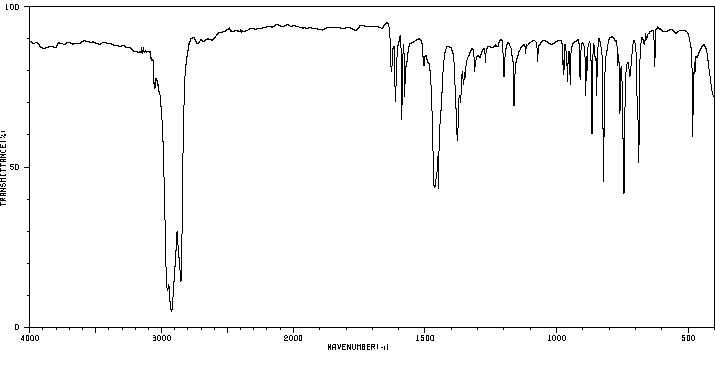
红外IR
-
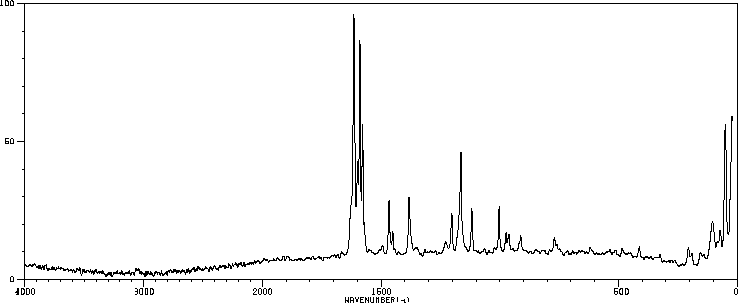
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮