6-溴-4-甲基苯并[b]噻吩-3(2H)-酮 | 5340-96-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.111
-
拓扑面积:48.5
-
氢给体数:1
-
氢受体数:2
SDS
反应信息
-
作为反应物:参考文献:名称:290.新的中间体和染料。第四部分 噻吩2-2-3-二羧酸酐与烃和酚的缩合摘要:DOI:10.1039/jr9560001429
-
作为产物:参考文献:名称:DE505159摘要:公开号:
文献信息
-
Georg kalischer and heinz scheyer申请人:——公开号:US01807693A1公开(公告)日:1931-06-02
311,208. Carpmael, A., (I. G. Farbenindustrie Akt.-Ges.). Feb. 6, 1928. Aldehydes; aldazines. - Aldehyde groups are introduced into cyclic compounds containing labile hydrogen atoms, in which other hydrogen atoms may be replaced by halogens or other monovalent substituents, except amino groups, by treatment with formamide or with derivatives thereof in which one of the hydrogen atoms of the amino group is substituted by aryl, and the other by alkyl, aralkyl on aryl, in the presence of an acid condensing agent containing chlorine, such as a chloride or oxychloride of phosphorus or sulphur or aluminium chloride. Suitable cyclic compounds are m-xylene, anthracene, N- ethylcarbazol, pyridine, quinoline, naphthostyril, naphtholethers, naphthalene, thioethers, oxythionaphthenes and anthrone and its nuclear substi. tution products. When using the formyl derivatives of secondary amines and cyclic compounds, such as oxythionaphthene and anthrone, containing in ring combination a methylene group and a Ketonic group capable of reacting in the tautomeric enolic form, chlorinated aldehydic derivatives are obtained, the hydroxyl group of the enolic form being replaced by chlorine. The reaction may be carried out in the presence of indifferent organic solvents. In examples, the following products are obtained, using formylmonomethylaniline and phosphorus oxychloride, in some cases in the presence of benzene : (1) panisaldehyde from anisol, (2) 2-ethoxy-1-naphthaldehyde from #-naphtholethylether, and 1-methoxy-5 : 6 : 7 : 8-tetrahydronaphthalene-4-aldehyde and 2-methoxy-5: 6 : 7 : 8-tetrahydronaphthalene. 1-aldehyde from 1- and 2-methoxy-5: 6 : 7 : 8. tetrahydronaphthalene, (3) anthracene-9-aldehyde from anthracene, and 1: 3-dimethyl-benzene-4- aldehyde from m-xylene, (4) a-chloranthracene-9- aldehyde from a-chloranthracene, and (5) 2- and 4-oxy-1-naphthaldehyde from #- and a-naphthol. In these examples, thionyl or sulphuryl chloride, or aluminium chloride may be used instead of phosphorus oxychloride, and other formylated secondary amines, such as formyldiphenylamine, or formamide, instead of formylmonomethylaniline. In a similar manner, 2: 7-dioxynaphthalene-1-aldehyde is obtained from 2: 7-dioxynaphthalene, 4: 8-dioxynaphthalene-1-aldehyde from 1: 5-dioxynaphthalene, 2-oxynaphthalene-1- aldehyde-3-carboxylic acid and the corresponding anilide from 2: 3-oxynaphthoic acid and its anilide, 2: 5-dimethyl-4-oxybenzaldehyde from pxylenol, 2: 4-dioxybenzaldehyde from resorcinol, and vanillin from guaiacol. In further examples, (6) 4-methyl-6-chlor-3-oxythionaphthene-2-aldehyde is obtained by heating 4-methyl-6-chlor-3- oxythionaphthene with formamide and anhydrous aluminium chloride or phosphorus oxychloride, (7) 6-ethoxy-3-oxythionaphthene-2-aldehyde is similarly obtained from 6-ethoxy-3-oxythionaphthene (8) N-ethylcarbazol is converted into an aldehyde by heating with formylmonomethylaniline and phosphorus oxychloride; pyridine and quinoline also yield aldehydes when similarly treated, and (9) naphthostyrilaldehyde is similarly obtained from naphthostyril. In further examples, in which chlorine is introduced at the same time as the aldehyde group, (10) 3-chlor-6-ethoxythionaphthene-2-aldehyde is obtained by treating 6. ethoxy-3-oxythionaphthene with formylmonomethylaniline and phosphorus oxychloride; 4- methyl-6-chlor-3-oxythionaphthene when similarly treated yields 4-methyl 3: 6 - dichlorthionaphthene-2-aldehyde, and (11) anthrone, when treated with formylmonomethylaniline or formyl. diphenylamine and phosphorus oxychloride yields 9-chloranthracene-10-aldehyde; when similarly treated, 2-chloranthrone yields 3: 10-dichloranthracene-9-aldehyde, 1 : 5-dichloranthrone yields 1: 5: 10-trichloranthracene-9-aldehyde, and desoxy-alizarine-dimethylether yields 1: 2-dimethnxy-lO-chloranthracene-9-nldehyde. The aldazines of most of the products are described.
311,208. Carpmael, A., (I. G. Farbenindustrie Akt.-Ges.). Feb. 6, 1928. 醛类化合物;醛肼。- 醛基被引入含有活泼氢原子的环状化合物中,其中其他氢原子可以被卤素或其他一价取代基(除氨基外)所取代,通过与甲酰胺或其衍生物处理,其中氨基的一个氢原子被芳基取代,另一个被烷基、芳基烷基或芳基取代,存在含氯的酸性缩合剂的情况下进行,如磷或硫或铝氯化物的氯化物或氧氯化物。适合的环状化合物是间二甲苯,蒽,N-乙基咔唑,吡啶,喹啉,萘基苯,萘醚,萘,硫醚,氧硫萘烯和蒽醌及其核取代产物。当使用二级胺和环状化合物的甲酰衍生物,如氧硫萘烯和蒽醌,其中环中组合了一个甲基和一个酮基,能够以互变式烯醇形式反应时,得到氯代醛衍生物,烯醇形式的羟基被氯取代。该反应可在惰性有机溶剂存在下进行。在示例中,使用甲酰单甲基苯胺和磷氧氯化物,在某些情况下,在苯的存在下,得到以下产品:(1)苯甲醛,(2)2-乙氧基-1-萘甲醛,从 #-萘醚,和 1-甲氧基-5:6:7:8-四氢萘-4-甲醛和 2-甲氧基-5:6:7:8-四氢萘烯-1-甲醛从 1-和 2-甲氧基-5:6:7:8-四氢萘烯,(3)蒽-9-甲醛从蒽,和 1:3-二甲基苯-4-甲醛从间二甲苯,(4)α-氯蒽-9-甲醛从α-氯蒽,和(5)2-和 4-氧基-1-萘甲醛从 #-和 α-萘酚。在这些示例中,可以使用亚硫酰氯或硫酰氯,或铝氯化物代替磷氧氯化物,以及其他甲酰化的二级胺,如甲酰二苯胺,或甲酰胺,代替甲酰单甲基苯胺。以类似的方式,从 2:7-二氧基萘烯得到 2:7-二氧基萘烯-1-甲醛,从 1:5-二氧基萘烯得到 4:8-二氧基萘烯-1-甲醛,从 2:3-氧基萘甲酸和其酰胺得到 2-氧基萘烯-1-甲醛-3-羧酸和相应的苯胺,从对甲酚得到 2:5-二甲基-4-氧基苯甲醛,从邻苯二酚得到 2:4-二氧基苯甲醛,从愈创木酚得到香草醛。在进一步的示例中,(6)4-甲基-6-氯-3-氧基硫萘烯-2-甲醛通过加热 4-甲基-6-氯-3-氧基硫萘烯与甲酰胺和无水氯化铝或磷氧氯化物得到,(7)6-乙氧基-3-氧基硫萘烯-2-甲醛同样是通过 6-乙氧基-3-氧基硫萘烯得到(8)N-乙基咔唑通过与甲酰单甲基苯胺和磷氧氯化物加热转化为醛;吡啶和喹啉同样在类似处理时产生醛,(9)萘基苯甲醛同样是从萘基苯甲醛中得到。在进一步的示例中,同时引入氯和醛基时,(10)3-氯-6-乙氧硫萘烯-2-甲醛通过处理 6-乙氧基-3-氧基硫萘烯与甲酰单甲基苯胺和磷氧氯化物得到;4-甲基-6-氯-3-氧基硫萘烯在类似处理时产生 4-甲基-3:6-二氯硫萘烯-2-甲醛,以及(11)蒽醌,当与甲酰单甲基苯胺或甲酰二苯胺和磷氧氯化物处理时得到 9-氯蒽-10-甲醛;类似处理时,2-氯蒽醌得到 3:10-二氯蒽-9-甲醛,1:5-二氯蒽醌得到 1:5:10-三氯蒽-9-甲醛,去氧基紫嫣甲醛二甲醚得到 1:2-二甲氧-10-氯蒽-9-醛。大多数产品的醛肼已被描述。 -
Verfahren zur Herstellung von 4,4'-Dimethyl-6,6'-dichlorthioindigo申请人:Clariant GmbH公开号:EP0933402A1公开(公告)日:1999-08-044,4'-Dimethyl-6,6'-dichlor-thioindigo wird in der stabilen Kristallmodifikation I, gekennzeichnet durch Beugungswinkel (2 Theta): Linien starker Intensität: 25,99; 27,26 Linien mittlerer Intensität: 10,27; 12,48; 22,39; 23,91; 24,82; 28,87, erhalten, indem man 3-Hydroxy-4-methyl-6-chlor-thionaphthen mit einer mittleren Teilchengröße von maximal 20 µm oxidiert.
-
237. The comparative reactivity of the carbonyl groups in the thionaphthenquinones. Part II. The influence of substituent groups in the thionaphthenquinones作者:Charles E. Dalgliesh、Frederick G. MannDOI:10.1039/jr9450000893日期:——
-
73. The comparative reactivity of the carbonyl groups in the thionaphthenquinones. Part I. The constitution of certain thioindigoid dyes作者:John Harley-Mason、Frederick G. MannDOI:10.1039/jr9420000404日期:——
-
134. Quinoxaline cyanines. Part IV. Some halogenated styryl derivatives作者:R. M. Anker、A. H. CookDOI:10.1039/jr9440000489日期:——
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
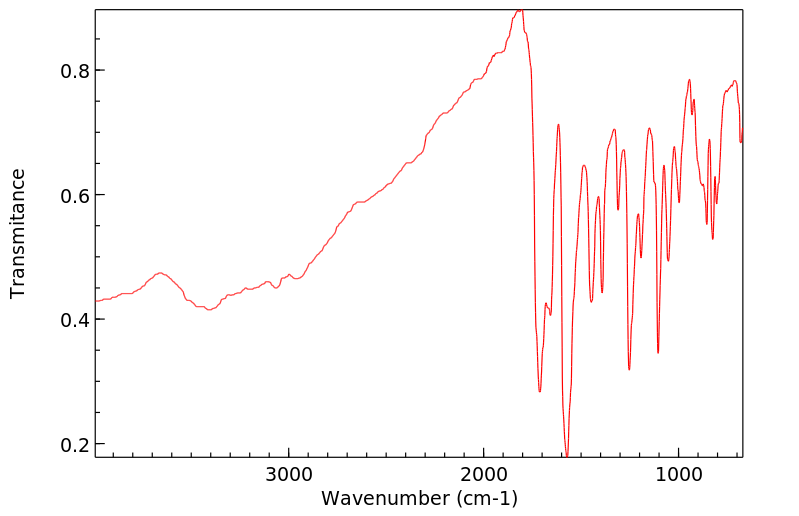
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息