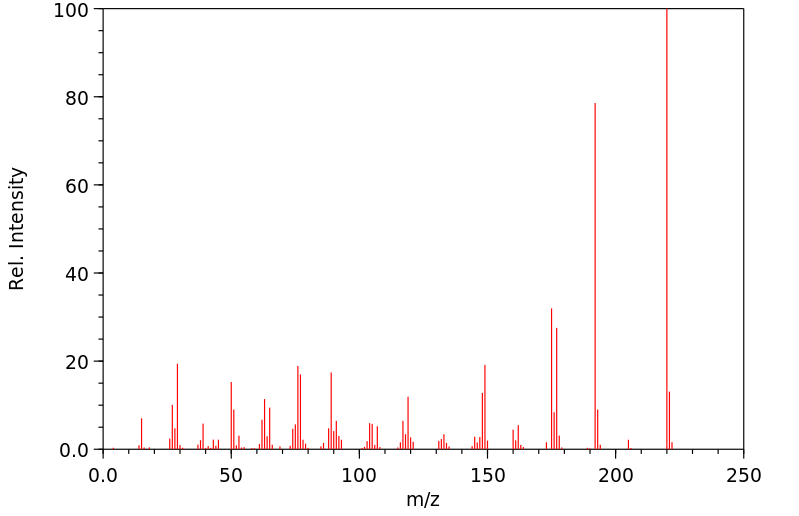
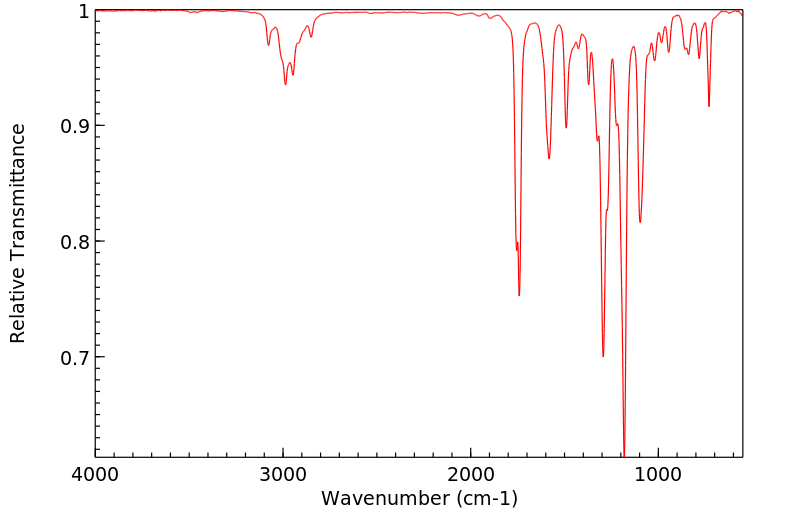
7-甲氧基苯并呋喃-2-甲酸乙酯 | 50551-58-1
中文名称
7-甲氧基苯并呋喃-2-甲酸乙酯
中文别名
4-甲氧基苯并呋喃-2-羧酸乙酯;7-甲氧基苯并呋喃-2-羧酸乙酯
英文名称
ethyl 7-methoxybenzofuran-2-carboxylate
英文别名
Ethyl 7-methoxy-1-benzofuran-2-carboxylate
CAS
50551-58-1
化学式
C12H12O4
mdl
MFCD00060510
分子量
220.225
InChiKey
SXCMZYVXJUJIDJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:85-90 °C
-
沸点:316.2±22.0 °C(Predicted)
-
密度:1.192±0.06 g/cm3(Predicted)
-
稳定性/保质期:
如果按照规定使用和存储,则不会分解,也没有已知的危险反应。
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:16
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:48.7
-
氢给体数:0
-
氢受体数:4
安全信息
-
安全说明:S24/25
-
海关编码:2932999099
-
危险性防范说明:P233,P260,P261,P264,P271,P280,P302+P352,P304,P304+P340,P305+P351+P338,P312,P321,P332+P313,P337+P313,P340,P362,P403,P403+P233,P405,P501
-
危险性描述:H315,H319,H335
-
储存条件:请将贮藏器密封并存放在阴凉、干燥处,同时确保工作环境有良好的通风或排气设施。
SDS
| Name: | Ethyl 7-methoxybenzofuran-2-carboxylate Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 50551-58-1 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 50551-58-1 | Ethyl 7-methoxybenzofuran-2-carboxylat | 97 | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. May be harmful if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, flush skin with plenty of water. Remove contaminated clothing and shoes. Get medical aid if irritation develops and persists. Wash clothing before reuse.
Ingestion:
If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 50551-58-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystals
Color: yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C12H12O4
Molecular Weight: 220.23
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 50551-58-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Ethyl 7-methoxybenzofuran-2-carboxylate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 50551-58-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 50551-58-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 50551-58-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— ethyl 7-hydroxy-2-benzofurancarboxylate 39543-86-7 C11H10O4 206.198 7-甲氧基苯并呋喃-2-甲酸 7-methoxybenzofuran-2-carboxylic acid 4790-79-8 C10H8O4 192.171 —— 2-carboxy-7-hydroxybenzofuran 4790-80-1 C9H6O4 178.144 —— 2-hydroxymethyl-7-methoxy-benzofuran 75566-54-0 C10H10O3 178.188 碘化N-[2-(乙酰氧基)乙基]-N,N-二甲基-3-(三甲基甲硅烷基)丙烷-1-铵 7-Hydroxy-2-hydroxymethyl-benzofuran 258872-65-0 C9H8O3 164.161 —— 2-(chloromethyl)-7-methoxybenzofuran 75566-55-1 C10H9ClO2 196.633
反应信息
-
作为反应物:描述:7-甲氧基苯并呋喃-2-甲酸乙酯 在 喹啉 、 N-溴代丁二酰亚胺(NBS) 、 水 、 铜 、 sodium hydroxide 作用下, 以 丙酮 、 乙腈 为溶剂, 反应 16.52h, 生成 4-bromo-7-methoxybenzofuran参考文献:名称:多取代的嘧啶类化合物作为mPGES-1抑制剂:在卡拉胶诱导的大鼠爪水肿中发现具有强抗炎作用的强效PGE2抑制剂。摘要:我们报道了对多取代嘧啶进行的广泛的构效关系优化,从而发现了5-丁基-4-(4-苄氧基苯基)-6-苯基嘧啶-2-胺及其二氟类似物。这些化合物是PGE 2产生的亚微摩尔抑制剂(IC 50低至12 nM)。为了确定抗炎嘧啶的分子靶标,我们进行了广泛的研究,包括酶法测定,同源性建模和对接。二氟类似物同时抑制花生四烯酸级联的两个关键酶,即mPGES-1和COX-2,其中mPGES-1的抑制是主要的作用机理。研究的其他嘧啶类是有效的mPGES-1抑制剂,没有观察到对COX-1 / 2酶的抑制作用。此外,在急性炎症模型中,两种最有效的化合物在体内被证明是有效的,将角叉菜胶诱导的大鼠爪水肿抑制了36%和46%。这项研究的有希望的结果值得对所选的抗炎候选药物进行进一步的临床前评估。DOI:10.1002/cmdc.202000258
-
作为产物:描述:(2-甲酰基-6-甲氧基苯氧基)乙酸乙酯 在 P(MeNCH2CH2)3N 作用下, 以 乙醇 为溶剂, 反应 3.0h, 以98%的产率得到7-甲氧基苯并呋喃-2-甲酸乙酯参考文献:名称:P(MeNCH2CH2)3N:合成苯并呋喃-2-羧酸取代乙酯的高效催化剂摘要:一种优化的方法,通过使用商业上可获得的P(MeNCH2CH2)3N作为催化剂(0.4当量),在70°C下反应3小时,从取代的2-甲酰基苯氧乙酸乙酯合成取代的乙基苯并呋喃-2-羧酸乙酯,产率高达80-99%。DOI:10.1055/s-2001-13377
文献信息
-
Derivatives of benzofuran or benzodioxole申请人:Kyowa Hakko Kogyo Co., Ltd.公开号:US06514996B2公开(公告)日:2003-02-04An oxygen-containing heterocyclic compound represented by following Formula (I): wherein R1 and R2 independently represent hydrogen, lower alkyl, cyano, —(CH2)n—E1—CO—G1 (wherein E1 represents a bond, O, or NH; and G1 represents hydrogen, substituted or unsubstituted lower alkyl, OR6, or NR7R8; and n represents an integer of 0 to 4), or the like; R1 and R2 are combined to represent a saturated carbon ring together with a carbon atom adjacent thereto; or R2, and R11 or R13 described below are combined to form a single bond; R3 represents hydrogen, phenyl, or halogen; R4 represents hydroxy, lower alkoxy, or the like; A represents —C(R9)(R10)— or O; B represents O, NR11, —C(R12)(R13)—, or —C(R14)(R15)—C(R16)(R17)—; D represents (i) —C(R18)(R19)—X— (wherein X represents —C(R21)(R22)—, S, or NR23), (ii) —C(R19a)═Y— [Y represents —C(R24)—Z— (wherein Z represents CONH, CONHCH2, or a bond), or N], or (iii) a bond; and R5 represents aryl, an aromatic heterocyclic group, cycloalkyl, pyridine-N-oxide, cyano, or lower alkoxycarbonyl; or pharmaceutically acceptable salts thereof.根据您的要求,以下是该化学公式(I)的中文翻译: 一个含氧杂环化合物,由以下公式(I)表示:其中R1和R2独立代表氢、低级烷基、氰基、—(CH2)n—E1—CO—G1(其中E1代表一个键、O或NH;G1代表氢、取代或未取代的低级烷基、OR6或NR7R8;n代表0到4的整数),或类似物;R1和R2共同代表与相邻碳原子一起的饱和碳环;或者R2与下面描述的R11或R13结合形成一个单键;R3代表氢、苯基或卤素;R4代表羟基、低级烷氧基或类似物;A代表—C(R9)(R10)—或O;B代表O、NR11、—C(R12)(R13)—或—C(R14)(R15)—C(R16)(R17)—;D代表(i)—C(R18)(R19)—X—(其中X代表—C(R21)(R22)—、S或NR23)、(ii)—C(R19a)═Y— [Y代表—C(R24)—Z—(其中Z代表CONH、CONH 或一个键)或N],或(iii)一个键;R5代表芳基、芳香杂环基、环烷基、吡啶-N-氧化物、氰基或低级烷氧基甲酸;或其药物可接受的盐。
-
Synthetic studies on selective adenosine A2A receptor antagonists. Part II: Synthesis and structure–activity relationships of novel benzofuran derivatives作者:Osamu Saku、Mayumi Saki、Masako Kurokawa、Ken Ikeda、Shin-ichi Uchida、Takuya Takizawa、Noriaki UesakaDOI:10.1016/j.bmcl.2010.04.058日期:2010.6Based on the previously reported lead compound, a series of benzofuran derivatives were prepared to study their antagonistic activities to A2A receptor. The replacement of the phenyl group at the 4-position with a heterocyclic ring improved the PK profile and aqueous solubility. From these studies, we discovered a potent new A2A antagonist, 12a, which has both a good oral bioavailability and in vivo
-
Novel tacrine–benzofuran hybrids as potential multi-target drug candidates for the treatment of Alzheimer’s Disease作者:Gaia Fancellu、Karam Chand、Daniel Tomás、Elisabetta Orlandini、Luca Piemontese、Diana F. Silva、Sandra M. Cardoso、Sílvia Chaves、M. Amélia SantosDOI:10.1080/14756366.2019.1689237日期:2020.1.1conjugates showed very good activity for AChE inhibition (sub-micromolar range) and good capacity for the inhibition of self- and Cu-mediated Aβ aggregation, with dependence on the linker size and substituent groups of each main moiety. Neuroprotective effects were also found for the compounds through viability assays of neuroblastoma cells, after Aβ1-42 induced toxicity. Structure-activity relationship analysis抽象的 为寻求对抗阿尔茨海默氏病(AD)的多靶点药物的广泛兴趣,基于众所周知的乙酰胆碱酯酶(AChE)抑制剂他克林(TAC)的重新定位,设计并开发了一系列新的杂种与苯并呋喃(BF)衍生物偶联。BF框架旨在赋予共轭分子以抑制AChE(双峰方式)和淀粉样β肽聚集的能力,此外还为具有羟基的杂合体提供金属(Fe,Cu)螯合能力和伴随的额外抗氧化活性。代换。新的TAC-BF共轭物显示出非常好的AChE抑制活性(亚微摩尔范围),并且具有抑制自身和Cu介导的Aβ聚集的能力,这取决于每个主要部分的接头大小和取代基。1-42诱导的毒性。结构-活性关系分析提供了最佳结构参数的见解,以考虑到未来在AD治疗中的潜在应用。
-
DERIVATIVES OF BENZOFURAN OR BENZODIOXOLE申请人:——公开号:US20020128290A1公开(公告)日:2002-09-12An oxygen-containing heterocyclic compound represented by following Formula (I): 1 wherein R 1 and R 2 independently represent hydrogen, lower alkyl, cyano, —(CH 2 ) n —E 1 —CO—G 1 (wherein E 1 represents a bond, O, or NH; and G 1 represents hydrogen, substituted or unsubstituted lower alkyl, OR 6 , or NR 7 R 8 ; and n represents an integer of 0 to 4), or the like; R 1 and R 2 are combined to represent a saturated carbon ring together with a carbon atom adjacent thereto; or R 2 , and R 11 or R 13 described below are combined to form a single bond; R 3 represents hydrogen, phenyl, or halogen; R 4 represents hydroxy, lower alkoxy, or the like; A represents —C(R 9 )(R 10 )— or O; B represents O, NR 11 , —C(R 12 )(R 13 )—, or —C(R 14 )(R 15 )—C(R 16 )(R 17 )—; D represents (i) —C(R 18 )(R 19 )—X— (wherein X represents —C(R 21 )(R 22 )—, S, or NR 23 ), (ii) —C(R 19a )═Y— [Y represents —C(R 24 )—Z— (wherein Z represents CONH, CONHCH 2 , or a bond), or N], or (iii) a bond; and R 5 represents aryl, an aromatic heterocyclic group, cycloalkyl, pyridine-N-oxide, cyano, or lower alkoxycarbonyl; or pharmaceutically acceptable salts thereof.以下是由下式(I)表示的含氧杂环化合物: 其中R1和R2独立地表示氢、较低的烷基、氰基、—(CH2)n—E1—CO—G1(其中E1表示键、O或NH;G1表示氢、取代或未取代的较低烷基、OR6或NR7R8;n表示0到4的整数),或类似物;R1和R2结合表示与相邻碳原子一起形成饱和碳环;或R2,以及下文描述的R11或R13结合形成单键;R3表示氢、苯基或卤素;R4表示羟基、较低的烷氧基或类似物;A表示—C(R9)(R10)—或O;B表示O、NR11、—C(R12)(R13)—或—C(R14)(R15)—C(R16)(R17)—;D表示(i)—C(R18)(R19)—X—(其中X表示—C(R21)(R22)—、S或NR23)、(ii)—C(R19a)═Y—[Y表示—C(R24)—Z—(其中Z表示CONH、CONH 或键),或N],或(iii)键;R5表示芳基、芳香杂环基、环烷基、吡啶-N-氧化物、氰基或较低烷氧羰基;或其药学上可接受的盐。
-
Benzofuran derivatives申请人:Kyowa Hakko Kogyo Co., Ltd.公开号:US06395738B1公开(公告)日:2002-05-28The present invention relates to benzofuran derivatives represented by following general formula (I): wherein R1 represents lower alkyl, R2 represents hydrogen or substituted or unsubstituted lower alkyl, R3, R4, R5 and R6 independently represent hydrogen or lower alkyl, X represents CH2 or C═O, and Y represents CH2 or NH, or pharmaceutically acceptable salts thereof.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
()-2-(5-甲基-2-氧代苯并呋喃-3(2)-亚乙基)乙酸乙酯
顺式-1-((2-(5-氯-2-苯并呋喃基)-4-甲基-1,3-二氧戊环-2-基)甲基)-1H-1,2,4-三唑
顺式-1-((2-(5,7-二氯-2-苯并呋喃基)-4-乙基-1,3-二氧戊环-2-基)甲基)-1H-咪唑
顺式-1-((2-(2-苯并呋喃基)-4-乙基-1,3-二氧戊环-2-基)甲基)-1H-1,2,4-三唑
霉酚酸酯杂质B
雷美替胺杂质3
雷美替胺杂质22
雷美替胺杂质
间甲酚紫
间甲基苯基(苯并呋喃-2-基)甲醇
长管假茉莉素C
钠1,4-二[(2-乙基己基)氧基]-1,4-二氧代-2-丁烷磺酸酯-3,3-二(4-羟基苯基)-2-苯并呋喃-1(3H)-酮(1:1:1)
金霉素
酪氨酸,b-羰基-
酞酸酐-d4
酚酞二丁酸酯
酚酞
酚红钠
酚红
邻苯二甲酸酐与马来酸酐,甘氨酰蜡素和二乙二醇的聚合物
邻苯二甲酸酐与己二醇的聚合物
邻苯二甲酸酐与三甘醇异壬醇的聚合物
邻苯二甲酸酐与2-乙基-2-羟甲基-1,3-丙二醇和2,5-呋喃二酮的聚合物
邻苯二甲酸酐与2-乙基-2-羟甲基-1,3-丙二醇、2,5-呋喃二酮和2-乙基己酸苯甲酸酯的聚合物
邻苯二甲酸酐-13C6
邻苯二甲酸酐-4-硼酸频哪醇酯
邻苯二甲酸酐,马来酸,二乙二醇,新戊二醇聚合物
邻甲酚酞二庚酸酯
邻甲酚酞二己酸酯
邻甲酚酞
贝康唑
表灰黄霉素
螺佐呋酮
螺[苯并呋喃-3(2H),4-哌啶]
螺[异苯并呋喃-1(3H),4’-哌啶]-3-酮
螺[异苯并呋喃-1(3H),4'-哌啶]-3-酮盐酸盐
螺[异苯并呋喃-1(3H),3’-吡咯烷]-3-酮
螺[1-苯并呋喃-2,1'-环丙烷]-3-酮
薄荷内酯
萘并[2,3-b]呋喃-8(4H)-酮,4a,5,6,7,8a,9-六氢-,顺-
莫罗卡尼
荨麻叶泽兰酮
荧光胺
苯酞-3-乙酸
苯酚,2-[3-(2-苯并呋喃基)-5,6-二氢-1,2,4-三唑并[3,4-b][1,3,4]噻二唑-6-基]-
苯酐二乙二醇共聚物
苯酐
苯甲酸,2-[(1,3-二羰基丁基)氨基]-,甲基酯
苯甲酸,2,2-二(羟甲基)丙烷-1,3-二醇,异苯并呋喃-1,3-二酮
苯甲酰氯化,3-甲氧基-4-甲基-