3-phenyl-2H-cyclohepta[b]furan-2-one | 33654-72-7
中文名称
——
中文别名
——
英文名称
3-phenyl-2H-cyclohepta[b]furan-2-one
英文别名
3-phenyl-cyclohepta[b]furan-2-one;3-Phenyl-1-oxoazulan-2-on;3-phenylcyclohepta[b]furan-2-one
CAS
33654-72-7
化学式
C15H10O2
mdl
——
分子量
222.243
InChiKey
JHBDEBOEPZMOBJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:517.3±29.0 °C(Predicted)
-
密度:1.25±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:17
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:描述:3-phenyl-2H-cyclohepta[b]furan-2-one 在 磷酸 、 硫酸 、 sodium ethanolate 、 亚硝酸异戊酯 作用下, 以 1,4-二氧六环 、 乙醇 为溶剂, 生成 1-phenylazulene参考文献:名称:由肌钙蛋白化合物合成 1-Phenylazulene 和 2-Phenylazulene摘要:1-苯基天青素 (I) 和 2-苯基天青素 (II) 由类肌醇化合物合成。3-苯基-(III)和3-苯甲酰基-2H-环庚[b]呋喃-2-酮(IV)分别通过2-氯托酮与苯乙酸乙酯和苯甲酰乙酸乙酯反应制备。III 与丙二腈或氰基乙酸乙酯反应生成 1-苯基芴衍生物,I 由其衍生。另一方面,IV与氰基乙酸乙酯或丙二酸二乙酯的反应得到2-苯基芴衍生物,从中衍生出II。DOI:10.1246/bcsj.44.2210
文献信息
-
Direct synthesis of 2-arylazulenes by [8+2] cycloaddition of 2<i>H</i>-cyclohepta[<i>b</i>]furan-2-ones with silyl enol ethers作者:Taku Shoji、Shuhei Sugiyama、Yoshiaki Kobayashi、Akari Yamazaki、Yukino Ariga、Ryuzi Katoh、Hiroki Wakui、Masafumi Yasunami、Shunji ItoDOI:10.1039/c9cc09376a日期:——We developed a procedure for the direct synthesis of 2-arylazulenes, which were obtained in moderate to excellent yields, by [8+2] cycloaddition of 2H-cyclohepta[b]furan-2-ones with aryl-substituted silyl enol ethers. The structures of some 2-arylazulenes were clarified by single-crystal X-ray analysis. The 2-phenylazulene derivatives obtained by this study showed noticeable fluorescence in acidic
-
A Facile Synthesis of 2-Substituted 2,3-Dihydro-4(1<i>H</i>)-Azuleno[2,1-<i>d</i>]Pyrimidinones作者:Dao-Lin Wang、Wei Li、Jiao Xu、Yuan-Feng Li、Shao-Fei Li、Li-Nan Lin、Kimiaki ImafukuDOI:10.1002/jhet.888日期:2016.11A facile, efficient, and novel approach to access 2‐substituted 2,3‐dihydro‐4(1H)‐azuleno[2,1‐d]pyrimidinones was developed by condensation of 2‐amino‐1‐carbamoyl‐3‐phenylazulene with ary1 aldehydes or ketones in ionic liquids by catalyzed p‐toluenesulfonic acid.
-
ROSEOBACTICIDES AND USES THEREOF申请人:Kolter Roberto公开号:US20130252815A1公开(公告)日:2013-09-26Embodiments of the invention relate to compounds and methods for controlling algal growth, for example, in bodies of water or surfaces exposed to algae. Provided are compounds having algicidal activities and methods of use of these compounds as well as formulations and compositions comprising the compound having algicidal activities.本发明实施例涉及化合物和方法,用于控制藻类生长,例如在水体或暴露于藻类的表面中。提供了具有藻类杀灭活性的化合物以及使用这些化合物的方法,以及包含具有藻类杀灭活性的化合物的配方和组合物。
-
US9131687B2申请人:——公开号:US9131687B2公开(公告)日:2015-09-15
表征谱图
-
氢谱1HNMR
-
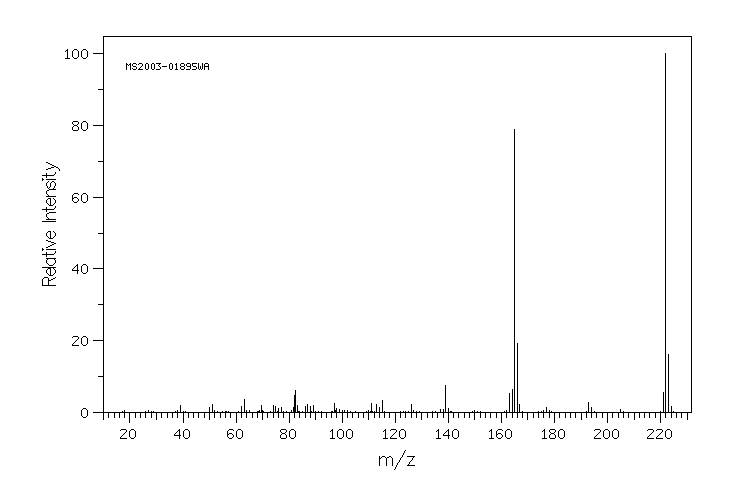
质谱MS
-
碳谱13CNMR
-
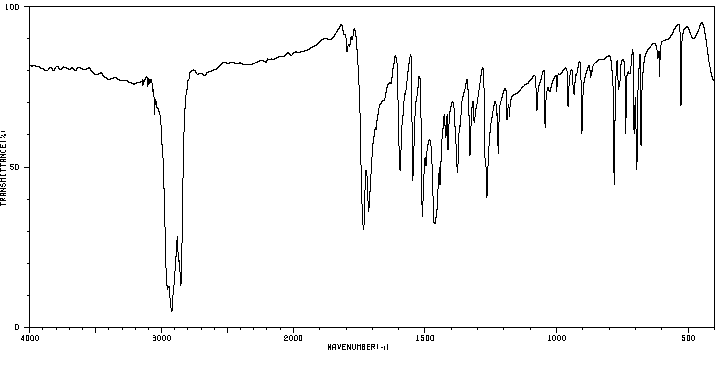
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
[2-二(2,4-二叔-丁基苯氧基)磷烷氧基-3,5-二叔-丁基-苯基]-氯-钯
N-(2-氰基乙基)-N-(2-吗啉-4-基乙基)-4-羰基-9,10-二氢-4H-苯并[4,5]环庚三烯并[1,2-b]呋喃-3-甲酰胺盐酸
N-(2-(二乙胺)乙基)-4-((2-(二乙胺)乙基)氨基)-9,10-二氢-4-羟基-4H-苯并(4,5)环庚三烯并[1,2-b]呋喃-3-甲酰胺
N,N-二乙基-4-羰基-9,10-二氢-4H-苯并[4,5]环庚三烯并[1,2-b]呋喃-3-甲酰胺
6H-2-氧杂薁-6-酮
5-甲基-2,3-二氢-7H-呋喃并[3,2-g]色烯-7-酮
5-异丙基-3-(甲氧羰基)-2H-环庚烷[b]呋喃-2-酮
3-乙酰基-2H-环庚并[b]呋喃-2-酮
3-(甲氧羰基)-2H-环庚[b]呋喃-2-酮
3,5,8-三甲基薁并[6,5-b]呋喃
2H-环戊并(b)呋喃-2-酮
2,7,8,9-四氢-6-甲基-9-亚甲基-2-氧代薁并[4,5-b]呋喃-3-甲醛
(8R)-1,5,8-三甲基-7,8-二氢-6H-薁并[7,6-D]呋喃-2-酮
(5aR,6S)-rel-(-)-5,5a,6,10-四氢-5a,6-二甲基-4H-苯并(5,6)环庚并(1,2-b)呋喃
(3R,5Z,7E)-3,25-二羟基-9,10-裂胆甾-5,7,10-三烯-23-酮
(2Z)-3-甲基-N-苯基-2H-环庚并[b]呋喃-2-亚胺
3-Methyl-8-hydroxy-2H-cycloheptafuran-2-on
5-bromo-1,3-di-tert-butyl-4,4,6-trimethyl-4H-cyclopenta[c]furan
2-Methyl-7-propylcycloheptafuran-8-on
4-methoxy-2,3,6-trimethyl-8,9-dihydro-furo[2,3-f]isoquinoline
3-(3,4-dihydro-2H-naphthalen-1-ylidenemethyl)furan
(4α,4aβ,5α,7α,7aα,8α,9β)-(+/-)-9-<<(1,1-dimethylethyl)dimethylsilyl>oxy>-4,4a,5,6,7,7a,8,9-octahydro-4a,8-dimethyl-4-(1-ethoxyethoxy)-5-<(2-methoxyethoxy)methoxy>-7-methoxyazuleno<6,5-b>furan
3-bromo-2-(4-fluorophenyl)-5,6-dihydro-4H-benzo[6,7]cyclohepta[1,2-b]furan
1-benzyl-3-ethyl-4,5,6,7-tetrahydroisobenzofuran
(E)-4,4,-dimethyl-3-(3,4-pentano-2-furyl)-2-pentenenitrile
(Z)-4,4-dimethyl-3-(3,4-pentano-2-furyl)-2-pentenenitrile
3,4,3',4'-tetrachloro-6,6'-o-phenylene-bis-pyran-2-one
4-(Furan-2-yl)-3,15-dioxatetracyclo[6.6.1.02,6.09,14]pentadeca-2(6),4,9,11,13-pentaene
4-Methoxy-2-methyl-6,7,8,9-tetrahydro-3-oxa-cyclohepta[e]inden-10-one
2-cyano-3-(5-isopropyl-2-oxo-2H-cyclohepta[b]furan-3-yl)-but-2-enedinitrile
3-cyano-2H-cycloheptafuran-2-one
7,7-Dimethyl-4,5,6,7,8,9-hexahydro-naphtho[2,3-c]furan-5-ol
5-Isopropyl-2-oxo-2H-cyclohepta[b]furan-3-carbonitrile
diethyl 1-phenyl-5-(5,6,7,8-tetrahydro-4H-cyclohepta[c]furan-3-yl)bicyclo[3.1.0]hexane-3,3-dicarboxylate
(4aS,7aS)-6,6-Dimethyl-8-methylene-4,4a,5,6,7,7a,8,9-octahydro-2-oxa-cyclopenta[f]azulene
4,5,6,7-tetrahydro-8H-cyclohepta[b]furan-8-one
ethyl 8-hydroxy-2-oxo-2H-cycloheptafuran-3-carboxylate
2,3-Pentamethylen-5-methoxy-benzofuran
8-Ethoxy-6-(4-methoxy-phenyl)-1,3-dimethyl-cyclohepta[c]furan-4-one
2-((3-((1-(furan-2-ylmethylene)-1H-inden-3-yl)methylene)-2,3-dihydro-1H-inden-1-ylidene)methyl)furan
isognididione
3β,9α,10β-Trihydroxyfuranoeremophilan
3-acetyl-7-isopropyl-cyclohepta[b]furan-2-one
2-(1-ethoxymethyleneamino)-4-(4-methoxyphenyl)-8,10-dimethyl-7-oxo-4H,7H-furo[3',4':6,7]cyclohepta[1,2-b]pyran-3-carbonitrile
furanoeremophilane-3β,9β,10β-triol