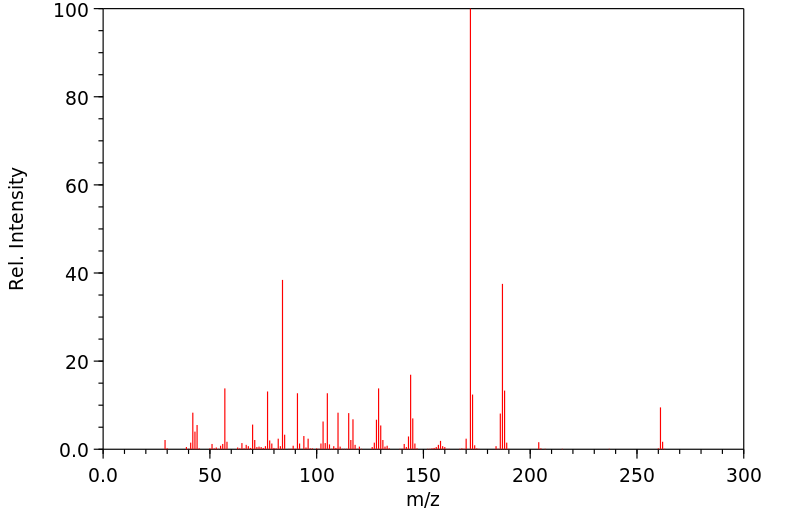
代谢
在给狗静脉注射后,从尿液中唯一可提取的代谢物是去甲阿尔法普罗定。
FOLLOWING IV ADMIN TO DOGS THE ONLY EXTRACTABLE METABOLITE FROM URINE WAS NORALPHAPRODINE.
来源:Hazardous Substances Data Bank (HSDB)