2,4-二硝基噻吩 | 5347-12-6
中文名称
2,4-二硝基噻吩
中文别名
——
英文名称
2,4-dinitrothiophene
英文别名
3,5-dinitrothiophene;2,4-dinitro-thiophene;2,4-Dinitro-thiophen;2.4-Dinitro-thiophen;2,4-Dinitrothiophen
CAS
5347-12-6
化学式
C4H2N2O4S
mdl
MFCD00039683
分子量
174.137
InChiKey
RZKBGZBDWAUWMC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:56°C
-
沸点:291.98°C (estimate)
-
密度:1.715 (estimate)
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:11
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:120
-
氢给体数:0
-
氢受体数:5
安全信息
-
海关编码:2934999090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氨基-3,5-二硝基噻吩 2-amino-3,5-dinitrothiophene 2045-70-7 C4H3N3O4S 189.152 —— 2-iodo-3,5-dinitrothiophene 54728-27-7 C4HIN2O4S 300.033
反应信息
-
作为反应物:参考文献:名称:Putochin; Sorokin, 1955, # 5, p. 261,263, 264摘要:DOI:
-
作为产物:描述:2-噻吩硼酸 在 dipotassium peroxodisulfate 、 bismuth (III) nitrate pentahydrate 作用下, 以 甲苯 为溶剂, 反应 12.0h, 以68%的产率得到2,4-二硝基噻吩参考文献:名称:IPSO芳基硼酸的-Nitration与硝酸铋和Perdisulfate摘要:的有效和一锅合成法本位的芳基硼酸-nitration已经研制成功。高效,普遍适用性以及包括杂环和官能团在内的更大的底物范围使该方法具有优势。由于其简单性,我们期望在合成中找到该方法的应用。DOI:10.1021/ol300325t
文献信息
-
A novel method for the oxidation of thiophenes. Synthesis of thiophene 1,1-dioxides containing electron-withdrawing substituents作者:V. G. Nenajdenko、A. M. Moiseev、E. S. BalenkovaDOI:10.1007/s11172-005-0107-9日期:2004.10A novel method for the synthesis of thiophene 1,1-dioxides by oxidation of substituted thiophenes with trifluoroperoxyacetic acid was developed. The effect of the solvent nature on the course of the reaction was studied and optimum conditions for the oxidation of thiophenes containing various functional groups were found. Previously unknown thiophene dioxides were obtained.
-
Palladium-Catalyzed Dearomative Trimethylenemethane Cycloaddition Reactions作者:Barry M. Trost、Veronika Ehmke、B. Michael O’Keefe、Dustin A. BringleyDOI:10.1021/ja5044825日期:2014.6.11exclusive formation of the dearomatized alicyclic products without subsequent rearomatization. The reaction is tolerant toward a broad range of heterocyclic and benzenoid substrates. The use of chiral bisdiamidophosphite ligands enabled the development of an enantioselective variant of this transformation, representing one of the rare examples of an asymmetric catalytic dearomatization process.
-
Ranking the Reactivity of Superelectrophilic Heteroaromatics on the Electrophilicity Scale作者:François Terrier、Sami Lakhdar、Taoufik Boubaker、Régis GoumontDOI:10.1021/jo0505526日期:2005.8.1ions. Such a ranking holds promise for expanding the range of coupling reactions which can be envisioned with such strongly electron-deficient neutral heteroaromatics as nitrobenzofuroxans and related compounds. Arguments are also given which exclude the possibility for the reactions studied to proceed via an electron-transfer mechanism.一系列参考碳亲核试剂,包括的反应的动力学Ñ -methylpyrrole甲,吲哚乙,Ñ甲基吲Ç,和烯胺d - g ^,用10缺电子芳族和杂芳族底物(1 - 10),从而导致在形成稳定的阴离子σ加合物时,已在20°C的乙腈中进行了研究。结果表明,与这些过程的碳-碳偶联步骤有关的二阶速率常数k 1很好地拟合了三参数方程log k(20°C)= s(Ñ + ë),允许亲电参数的确定ë的1 - 10,因此,通过迈尔等人用于阳离子电体限定在全面亲电尺度这些中性缺电子化合物的排名。(迈尔,H。;肯普夫,B,; Ofial,AR度Acc。化学式RES。2003,36,66)。所述Ë的值1 - 10被发现覆盖范围从-13至-5,由1,3,5-三硝基苯要去1中,至少活性分子,以4,6- dinitrotetrazolo [1,5-一个]吡啶8,4-硝基-6-三氟甲磺酰基苯并呋喃3和4,6-二硝基苯并呋喃2这
-
Studies on the biological activity of some nitrothiophenes作者:John O. Morley、Thomas P. MatthewsDOI:10.1039/b514441h日期:——inhibitory concentration required to inhibit the growth of E. coli, M. luteus and A. niger. The series displays a wide range of activities with 2-chloro-3,5-dinitrothiophene (3a) or 2-bromo-3,5-dinitrothiophene (3c) showing the highest activity against all three organisms, while the simplest compound of the series, 2-nitrothiophene (3s) shows the smallest activity in each case. The mode of action of 3a and
-
The ambident electrophilic behavior of 5-nitro-3-X-thiophenes in σ-complexation processes作者:Wahiba Gabsi、Khalid Essalah、Régis Goumont、Bahoueddine Tangour、Taoufik BoubakerDOI:10.1002/kin.21190日期:2018.9terms of Brønsted relationships reveals that the reaction mechanism likely involves a single‐electron transfer (SET) process. The excellent correlations upon plotting the rate constants versus the oxidation potentials Eo values is an additional evidence that reactions between thiophenes and phenoxide anions are proceeding through an initial electron transfer. It is of particular interest to note that the在20°C的水溶液中研究了3,5-二硝基噻吩1和3-氰基-5-硝基噻吩2与一系列对位取代的酚盐阴离子3a–c的反应动力学。已确定两个噻吩的两个未取代的亲电子中心(C(2)和C(4))。Fukui函数正确地将C(2)和C(4)原子预测为这些电子不足的噻吩1和2的最亲电子中心。根据布朗斯台德关系对实验数据进行分析表明,反应机理可能涉及单电子转移(SET)过程。绘制速率常数与氧化电位E o时的极好的相关性值是噻吩和酚盐阴离子之间的反应正在通过初始电子转移进行的另一个证据。特别值得注意的是,本文研究的系统提供了σ络合反应中SET机理的罕见例子。根据自由能关系日志ķ =小号(Ñ + ë)(Angew化学杂志,诠释。编英格兰,1994,33,938-957),亲电性参数È已经确定了噻吩的C-4和C-2位置,并将其与其他环境亲电试剂的反应性进行了比较。另一方面,这些噻吩与氢氧根离子反应的二阶速率常数已在
表征谱图
-
氢谱1HNMR
-
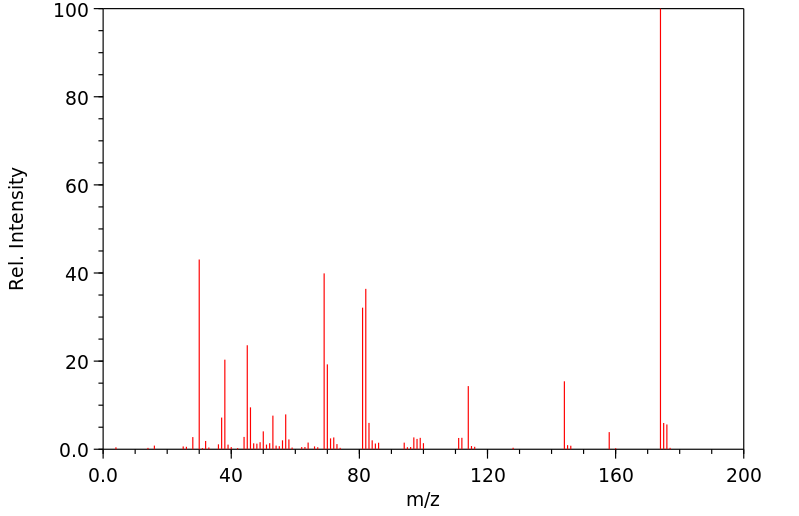
质谱MS
-
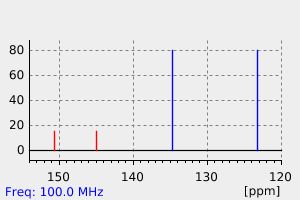
碳谱13CNMR
-
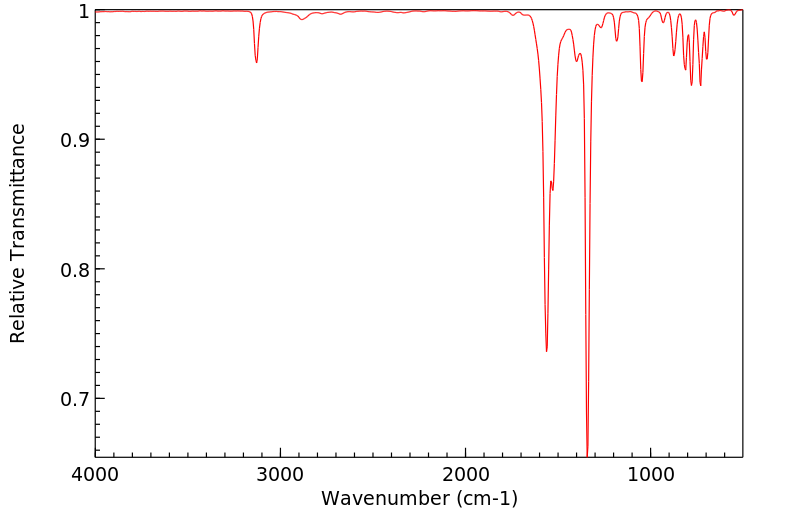
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
阿罗洛尔
阿替卡因
阿克兰酯
锡烷,(5-己基-2-噻吩基)三甲基-
邻氨基噻吩(2盐酸)
辛基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
辛基4,6-二溴噻吩并[3,4-b]噻吩-2-羧酸酯
辛基2-甲基异巴豆酸酯
血管紧张素IIAT2受体激动剂
葡聚糖凝胶LH-20
苯螨噻
苯并[c]噻吩-1-羧酸,5-溴-4,5,6,7-四氢-3-(甲硫基)-4-羰基-,乙基酯
苯并[b]噻吩-2-胺
苯并[b]噻吩-2-胺
苯基-[5-(4,4,5,5-四甲基-[1,3,2]二氧杂硼烷-2-基)-噻吩-2-基亚甲基]-胺
苯基-(5-氯噻吩-2-基)甲醇
苯乙酸,-α--[(1-羰基-2-丙烯-1-基)氨基]-
苯乙酰胺,3,5-二氨基-a-羟基-2,4,6-三碘-
苯乙脒,2,6-二氯-a-羟基-
腈氨噻唑
聚(3-丁基噻吩-2,5-二基),REGIOREGULAR
硝呋肼
硅烷,(3-己基-2,5-噻吩二基)二[三甲基-
硅噻菌胺
盐酸阿罗洛尔
盐酸阿罗洛尔
盐酸多佐胺
甲酮,[5-(1-环己烯-1-基)-4-(2-噻嗯基)-1H-吡咯-3-基]-2-噻嗯基-
甲基5-甲酰基-4-甲基-2-噻吩羧酸酯
甲基5-乙氧基-3-羟基-2-噻吩羧酸酯
甲基5-乙基-3-肼基-2-噻吩羧酸酯
甲基5-(氯甲酰基)-2-噻吩羧酸酯
甲基5-(氯乙酰基)-2-噻吩羧酸酯
甲基5-(氨基甲基)噻吩-2-羧酸酯
甲基5-(4-甲氧基苯基)-2-噻吩羧酸酯
甲基5-(4-甲基苯基)-2-噻吩羧酸酯
甲基5-(1,3-二氧戊环-2-基)-2-噻吩羧酸酯
甲基4-硝基-2-噻吩羧酸酯
甲基4-氰基-5-(4,6-二氨基吡啶-2-基)偶氮-3-甲基噻吩-2-羧酸酯
甲基4-氨基-5-(甲硫基)-2-噻吩羧酸酯
甲基4-{[(2E)-2-(4-氰基苯亚甲基)肼基]磺酰}噻吩-3-羧酸酯
甲基4-(氯甲酰基)-3-噻吩羧酸酯
甲基4-(氨基磺酰基氨基)-3-噻吩羧酸酯
甲基3-甲酰氨基-4-甲基-2-噻吩羧酸酯
甲基3-氨基-5-异丙基-2-噻吩羧酸酯
甲基3-氨基-5-(4-溴苯基)-2-噻吩羧酸酯
甲基3-氨基-4-苯基-5-(三氟甲基)-2-噻吩羧酸酯
甲基3-氨基-4-氰基-5-甲基-2-噻吩羧酸酯
甲基3-氨基-4-丙基-2-噻吩羧酸酯
甲基3-[[(4-甲氧基苯基)亚甲基氨基]氨基磺酰基]噻吩-2-羧酸酯