顺式-1,2-二氯环己烷 | 10498-35-8
中文名称
顺式-1,2-二氯环己烷
中文别名
——
英文名称
cis-1,2-dichloro-cyclohexane
英文别名
cis-1,2-Dichlorcyclohexan;cis-1,2-Dichlorocyclohexan;cis-1,2-Dichlorocyclohexane;(1S,2R)-1,2-dichlorocyclohexane
CAS
10498-35-8
化学式
C6H10Cl2
mdl
——
分子量
153.051
InChiKey
GZEZIBFVJYNETN-OLQVQODUSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-1.5°C
-
沸点:181.5°C (rough estimate)
-
密度:1.2021
-
保留指数:1092;1092;1094
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903890090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Parnes, Z. N.; Romanova, V. S.; Vol'pin, M. E., Journal of Organic Chemistry USSR (English Translation), 1988, p. 254 - 257摘要:DOI:
-
作为产物:参考文献:名称:Beger, J.; Jacobi, R.; Storch, A., Journal fur praktische Chemie (Leipzig 1954), 1980, vol. 322, # 3, p. 394 - 400摘要:DOI:
文献信息
-
The Electrochemical <i>cis</i> ‐Chlorination of Alkenes作者:Julia Strehl、Cornelius Fastie、Gerhard HiltDOI:10.1002/chem.202103316日期:2021.12.9Double role: The cis-chlorination of alkenes is realised in an electrochemical setup with PhSeCl as catalyst. The supporting electrolyte (TBACl) acts as nucleophilic reagent for the SN2-type replacement of selenium versus chloride in the key step of the process while the PhSeCl-alkene adduct is activated by electrochemically generated PhSeCl3.
-
Catalytic, stereospecific syn-dichlorination of alkenes作者:Alexander J. Cresswell、Stanley T.-C. Eey、Scott E. DenmarkDOI:10.1038/nchem.2141日期:2015.2As some of the oldest organic chemical reactions known, the ionic additions of elemental halogens such as bromine and chlorine to alkenes are prototypical examples of stereospecific reactions, typically delivering vicinal dihalides resulting from anti-addition. Although the invention of enantioselective variants is an ongoing challenge, the ability to overturn the intrinsic anti-diastereospecificity作为已知的一些最古老的有机化学反应,元素卤素如溴和氯在烯烃上的离子加成是立体有择反应的典型实例,通常递送由抗加成反应产生的邻位二卤化物。虽然对映选择性变异的发明是一项持续的挑战,颠覆固有的能力,抗这些变化的-diastereospecificity也是一个主要未解决的问题。在这里,我们描述了使用基于氧化还原活性主族元素(硒)的基团转移催化剂进行的烯烃的第一催化,顺式立体定向二氯化。以二苯基二硒化物(PhSeSePh)(5 mol%)作为前催化剂,苄基三乙基氯化铵(BnEt 3NCl)作为氯化物来源,N-氟吡啶鎓盐作为氧化剂,各种功能化的环状和无环1,2-二取代烯烃,包括简单的烯丙基醇,可提供具有精确立体控制的合成二氯化物。该方法有望在简化多氯天然产物(如氯磺脂)的合成中找到应用。
-
Peroxide-induced α-elimination of organic halides from organotellurium(<scp>IV</scp>) halides作者:Sakae Uemura、Shin-Ichi FukuzawaDOI:10.1039/c39800001033日期:——Treatment of organotellurium(IV) halides with t-butyl hydroperoxide in 1,4-dioxan, acetic acid, or acetonitrile affords the corresponding organic halides in good to moderate yields presumably as the result of a 1,2-halogen shift.
-
Chlorination of cyclohexene by copper(II) chloride作者:Philip H. Gamlen、Michael S. Henty、Hugh L. RobertsDOI:10.1039/dt9830001373日期:——Evidence is presented which indicates that CuCl+ is the reactive species in the chlorination of cyclohexene by copper(II) chloride in acetonitrile. The reaction is retarded by an excess of chloride ion and by copper(I) salts, and accelerated by aluminium trichloride. A kinetic scheme which accounts for these observations is suggested.
-
Organoselenium-catalyzed vicinal dichlorination of unsaturated phosphonates作者:Xianghua Zeng、Chunhua Gong、Junyong Zhang、Jingli XieDOI:10.1039/c6nj00658b日期:——Diphenyl diselenide (PhSeSePh) was effective as a pre-catalyst for the vicinal dichlorination of α,β-unsaturated phosphonates with sulfuryl chloride. The reaction conditions were mild and the desired products were formed in up to 93% yield and moderate diastereoselectivity (10 : 1). The important diphenylselenium dichloride intermediate was obtained and characterized by X-ray crystallography.
表征谱图
-
氢谱1HNMR
-
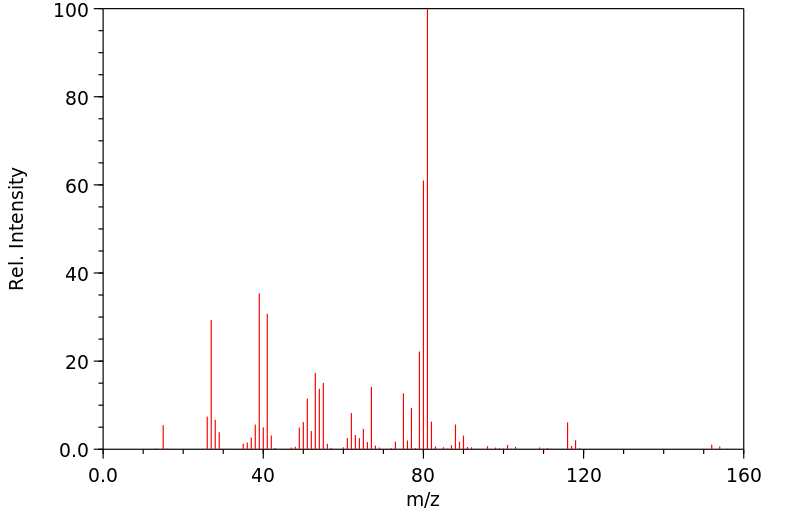
质谱MS
-
碳谱13CNMR
-
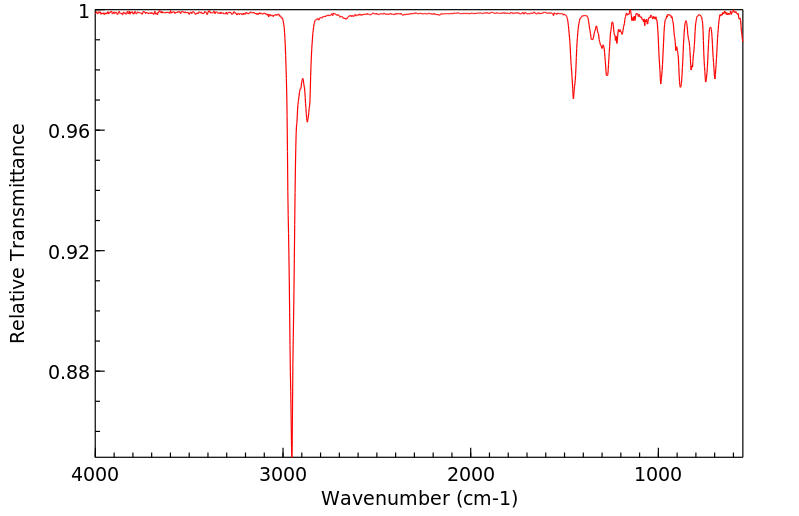
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-氯环己基高氯酸盐
顺式-1-溴-2-氟-环己烷
顺式-1-叔丁基-4-氯环己烷
顺式-1,2-二氯环己烷
顺-1H,4H-十二氟环庚烷
镓,三(三氟甲基)-
镁二(1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-十七氟-1-辛烷磺酸酯)
铵2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,12-二十三氟十二烷酸盐
铜N-(2-氨基乙基)乙烷-1,2-二胺2-氰基胍二氯化盐酸
钾{[(十七氟辛基)磺酰基](甲基)氨基}乙酸酯
钠3-[(3-{[(十七氟辛基)磺酰基]氨基}丙基)(甲基)氨基]-1-丙烷磺酸酯
重氮基烯,(1-溴环己基)(1,1-二甲基乙基)-,1-氧化
辛酸,十五氟-,2-(1-羰基辛基)酰肼
赖氨酰-精氨酰-精氨酰-苯基丙氨酰-赖氨酰-赖氨酸
诱蝇羧酯B1
诱蝇羧酯
萘并[2,1-b]噻吩-1(2H)-酮
膦基硫杂酰胺,P,P-二(三氟甲基)-
脲,N-(4,5-二甲基-4H-吡唑-3-基)-
肼,(3-环戊基丙基)-,盐酸(1:1)
组织蛋白酶R
磷亚胺三氯化,(三氯甲基)-
碳标记全氟辛酸
碘甲烷与1-氮杂双环(4.2.0)辛烷高聚合物的化合物
碘甲烷-d2
碘甲烷-d1
碘甲烷-13C,d3
碘甲烷
碘环己烷
碘仿-d
碘仿
碘乙烷-D1
碘[三(三氟甲基)]锗烷
硫氰酸三氯甲基酯
甲烷,三氯氟-,水合物
甲次磺酰胺,N,N-二乙基-1,1,1-三氟-
甲次磺酰氯,氯二[(三氟甲基)硫代]-
甲基碘-12C
甲基溴-D1
甲基十一氟环己烷
甲基丙烯酸正乙基全氟辛烷磺
甲基三(三氟甲基)锗烷
甲基[二(三氟甲基)]磷烷
甲基1-氟环己甲酸酯
环戊-1-烯-1-基全氟丁烷-1-磺酸酯
环己烷甲酸4,4-二氟-1-羟基乙酯
环己烷,1-氟-2-碘-1-甲基-,(1R,2R)-rel-
环己基五氟丙烷酸酯
环己基(1-氟环己基)甲酮
烯丙基十七氟壬酸酯