5-乙基-2-甲基哌啶 | 104-89-2
中文名称
5-乙基-2-甲基哌啶
中文别名
——
英文名称
5-ethyl-2-methylpiperidine
英文别名
2-methyl-5-ethyl piperidine;2-methyl-5-ethylpiperidine;5-ethyl-2-methyl-piperidine;5-Aethyl-2-methyl-piperidin;Kopellidin;2-Methyl-5-aethyl-piperidin
CAS
104-89-2
化学式
C8H17N
mdl
——
分子量
127.23
InChiKey
XOFNHZHCGBPVGJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-1°C (estimate)
-
沸点:156.06°C (estimate)
-
密度:0.8455 (estimate)
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:12
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:Xi
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,2-Dimethyl-5-ethyl-piperidin 6012-19-7 C9H19N 141.257
反应信息
-
作为反应物:描述:参考文献:名称:Levy, Chemische Berichte, 1895, vol. 28, p. 2273摘要:DOI:
-
作为产物:描述:参考文献:名称:Akkerman et al., Recueil des Travaux Chimiques des Pays-Bas, 1951, vol. 70, p. 899,913摘要:DOI:
文献信息
-
Heterocyclics useful as fungicides and fungicidal compositions thereof申请人:Hoffmann-La Roche Inc.公开号:US04241058A1公开(公告)日:1980-12-23Heterocyclic compounds characterized by the formula ##STR1## wherein R.sub.1, R.sub.3, R.sub.4, R.sub.5, R.sub.6, X and z are as hereinafter set forth, prepared, inter alia, by reacting a compound characterized by the formula ##STR2## with an amine characterized by the formula ##STR3## wherein R.sub.1, R.sub.3, R.sub.4, R.sub.5, R.sub.6, X and Y are as hereinafter set forth, are described. The end products are useful as fungicidal agents.以公式##STR1##为特征的杂环化合物,其中R1、R3、R4、R5、R6、X和Z如后文所述,通过至少与一种公比为##STR2##的化合物与一种公比为##STR3##的胺反应而制备,其中R1、R3、R4、R5、R6、X和Y如后文所述。所述最终产品可用作杀真菌剂。
-
Mannich base derivatives of 3-hydroxy-6- methyl-4H-pyran-4-one with antimicrobial activity作者:DEMET US、BARKIN BERK、ENİSE ECE GÜRDAL、NİHAN AYTEKİN、ZÜHTÜ TANIL KOCAGÖZ、BERRAK ÇAĞLAYAN、IŞIL KURNAZ、DİLEK DEMİR EROLDOI:10.3906/kim-0908-214日期:——A series of 3-hydroxy-6-methyl-2-[(substitutedpiperidine-1-yl) methyl]-4H-pyran-4-one structured compounds were synthesized by reacting 5-hydroxy-2-methyl-4H-pyran-4-one with suitable piperidine derivatives using Mannich reaction conditions. Antibacterial activities of the compounds for E. coli ATCC 25922, S. paratyphi ATCC BAA-1250, S. flexneri ATCC 12022, E. gergoviae ATCC 33426, and M. smegmatis ATCC 14468 were assessed in vitro by the broth dilution method for determination of minimum inhibitory concentration (MIC). In addition, their inhibitory effects over DNA gyrase enzyme were evaluated using a DNA gyrase supercoiling assay. All the synthesized compounds showed a MIC value of either 8 or 16 \mu g/mL for M. smegmatis, whereas minimum to moderate activity was achieved for the others. Those tested in the supercoiling assay had at best a very mild inhibition of the enzyme. This series deserves further attention for testing over Mycobacterium species and topoisomerase II inhibition to develop new lead drugs to treat non-tuberculous mycobacterial infections.通过在曼尼希反应条件下将5-羟基-2-甲基-4H-吡喃-4-酮与适宜的哌啶衍生物反应,合成了一系列具有3-羟基-6-甲基-2-[(取代哌啶-1-基)甲基]-4H-吡喃-4-酮结构的化合物。通过肉汤稀释法在体外评估了这些化合物对大肠杆菌ATCC 25922、沙门氏菌副伤寒亚种ATCC BAA-1250、福氏志贺菌ATCC 12022、杰戈维亚埃希氏菌ATCC 33426和耻垢分枝杆菌ATCC 14468的抗菌活性,以确定最小抑制浓度(MIC)。此外,还使用DNA促旋酶超螺旋测定法评估了它们对DNA促旋酶的抑制效果。所有合成的化合物对耻垢分枝杆菌的MIC值均为8或16 μg/mL,而对其他菌种则表现出最低至中等的活性。在超螺旋测定中测试的这些化合物对酶的最大抑制作用也是极其轻微的。这一系列化合物值得进一步关注,用于测试对分枝杆菌属物种和拓扑异构酶II的抑制作用,以开发治疗非结核性分枝杆菌感染的新型先导药物。
-
[EN] BENZOXAZEPINES AS INHIBITORS OF PI3K/m TOR AND METHODS OF THEIR USE AND MANUFACTURE<br/>[FR] BENZOXAZÉPINES COMME INHIBITEURS DE PI3K/M TOR, MÉTHODES D'UTILISATION ET DE FABRICATION BENZOXAZEPINES AS INHIBITORS OF PI3K/M TOR AND METHODS OF THEIR USE AND MANUFACTURE申请人:EXELIXIS INC公开号:WO2010138487A1公开(公告)日:2010-12-02The invention is directed to Compounds of Formula (I): the invention provides compounds that inhibit, regulate, and/or modulate P13K and/or mTOR that are useful in the treatment of hyperproliferative diseases, such as cancer, in mammals. This invention also provides methods of making the compound methods of using such compounds in the treatment of hyperproliferative diseases in mammals, especially humans, and to pharmaceutical compositions containing such compounds. For example, cancer in which activity against PI3fC-alph mTOR, or both contributes to its pathology and/or symptomatology include breast cancer mantle cell lymphoma, renal cell carcinoma, acute myelogenous leukemia, chronic myelogenous leukemia, NPM/ALK- transformed anaplastic large cell lymphoma, diffu large B cell lymphoma, rhabdomyosarcoma, ovarian cancer, endometrial cancer, cervic cancer, non small cell lung carcinoma, small cell lung carcinoma, adenocarcinoma, col cancer, rectal cancer, gastric carcinoma, hepatocellular carcinoma, melanoma, pancreat cancer, prostate carcinoma, thyroid carcinoma, anaplastic large cell lymphoma, hemangiom glioblastoma, or head and neck cancer.这项发明涉及式(I)的化合物:该发明提供了抑制、调节和/或调节P13K和/或mTOR的化合物,这些化合物在治疗哺乳动物的高增殖性疾病,如癌症,方面非常有用。该发明还提供了制备该化合物的方法,以及在治疗哺乳动物,特别是人类的高增殖性疾病中使用这些化合物的方法,以及含有这些化合物的药物组合物。例如,对PI3fC-α mTOR或两者都具有活性有助于其病理学和/或症状学的癌症包括乳腺癌、套细胞淋巴瘤、肾细胞癌、急性髓细胞白血病、慢性髓细胞白血病、NPM/ALK转化的间变性大细胞淋巴瘤、弥漫性大B细胞淋巴瘤、横纹肌肉瘤、卵巢癌、子宫内膜癌、宫颈癌、非小细胞肺癌、小细胞肺癌、腺癌、结肠癌、直肠癌、胃癌、肝细胞癌、黑色素瘤、胰腺癌、前列腺癌、甲状腺癌、间变性大细胞淋巴瘤、血管瘤、胶质母细胞瘤或头颈癌。
-
Anti-Viral Compounds申请人:DeGoey David A.公开号:US20100168138A1公开(公告)日:2010-07-01Compounds effective in inhibiting replication of Hepatitis C virus (“HCV”) are described. This invention also relates to processes of making such compounds, compositions comprising such compounds, and methods of using such compounds to treat HCV infection.描述了一种有效抑制丙型肝炎病毒(“HCV”)复制的化合物。本发明还涉及制备这种化合物的方法、包含这种化合物的组合物,以及使用这种化合物治疗HCV感染的方法。
-
SUBSTITUTED AZOLE AROMATIC HETEROCYCLES AS INHIBITORS OF 11BETA-HSD-1申请人:Bartberger D. Michael公开号:US20080021022A1公开(公告)日:2008-01-24Compounds of formula I and IV are described and have therapeutic utility, particularly in the treatment of diabetes, obesity and related conditions and disorder: wherein the variables A-B, R 1 , R 2 , m, and Q are described herein.化合物的公式I和IV已经描述,并具有治疗效用,特别是在治疗糖尿病、肥胖和相关疾病和紊乱方面: 其中变量A-B、R1、R2、m和Q在此处描述。
表征谱图
-
氢谱1HNMR
-
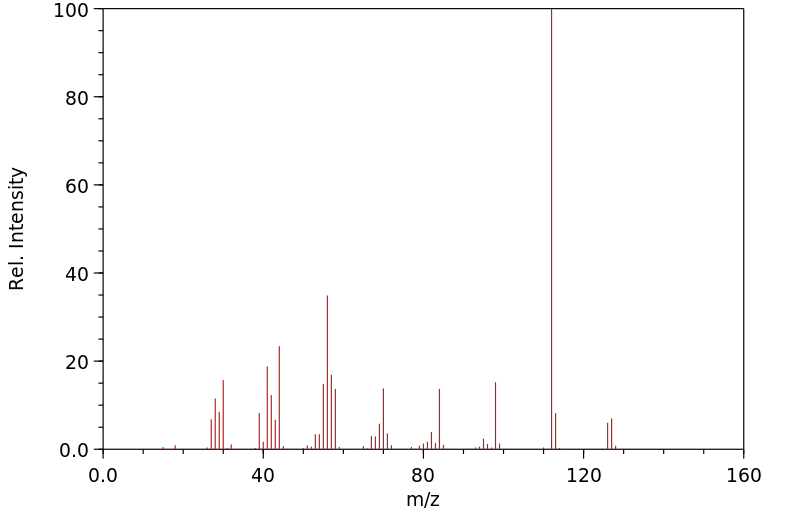
质谱MS
-
碳谱13CNMR
-
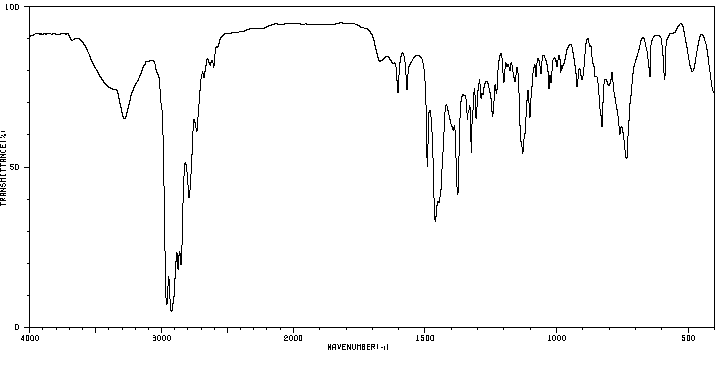
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-甲基哌啶盐酸盐;
(R)-2-苄基哌啶-1-羧酸叔丁酯
((3S,4R)-3-氨基-4-羟基哌啶-1-基)(2-(1-(环丙基甲基)-1H-吲哚-2-基)-7-甲氧基-1-甲基-1H-苯并[d]咪唑-5-基)甲酮盐酸盐
高氯酸哌啶
高托品酮肟
马来酸帕罗西汀
颜料红48:4
顺式3-氟哌啶-4-醇盐酸盐
顺式2,6-二甲基哌啶-4-酮
顺式1-苄基-4-甲基-3-甲氨基-哌啶
顺式-叔丁基4-羟基-3-甲基哌啶-1-羧酸酯
顺式-6-甲基-哌啶-1,3-二甲酸1-叔丁酯
顺式-5-(三氟甲基)哌啶-3-羧酸甲酯盐酸盐
顺式-4-叔丁基-2-甲基哌啶
顺式-4-Boc-氨基哌啶-3-甲酸甲酯
顺式-4-(氮杂环丁烷-1-基)-3-氟哌
顺式-3-顺式-4-氨基哌啶
顺式-3-甲氧基-4-氨基哌啶
顺式-3-BOC-3,7-二氮杂双环[4.2.0]辛烷
顺式-3-(1-吡咯烷基)环丁腈
顺式-3,5-哌啶二羧酸
顺式-3,4-二溴-3-甲基吡咯烷盐酸盐
顺式-2,6-二甲基-4-氧代哌啶-1-羧酸叔丁基酯
顺式-1-叔丁氧羰基-4-甲基氨基-3-羟基哌啶
顺式-1-boc-3,4-二氨基哌啶
顺式-1-(4-叔丁基环己基)-4-苯基-4-哌啶腈
顺式-1,3-二甲基-4-乙炔基-6-苯基-3,4-哌啶二醇
顺-4-(4-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-4-(2-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-3-氨基-4-氟哌啶-1-羧酸叔丁酯
顺-1-苄基-4-甲基哌啶-3-氨基酸甲酯盐酸盐
非莫西汀
雷芬那辛
雷拉地尔
阿维巴坦中间体4
阿格列汀杂质
阿尼利定盐酸盐 CII
阿尼利定
阿塔匹酮
阿哌沙班杂质BMS-591455
阿哌沙班杂质87
阿哌沙班杂质52
阿哌沙班杂质51
阿哌沙班杂质5
阿哌沙班杂质
阿哌沙班杂质
阿哌沙班-d3
阿哌沙班
阻聚剂701
间氨基谷氨酰胺