氧化铅 | 1317-36-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:886 °C(lit.)
-
沸点:1470 °C
-
密度:9.53
-
溶解度:溶于浓碱、盐酸和氯化铵。不溶于稀碱和醇。
-
暴露限值:a/nm
-
物理描述:Litharge appears as odorless gray or yellow green or red-brown solid. Sinks in water. (USCG, 1999)
-
颜色/状态:Exists in 2 forms: red to reddish-yellow, tetragonal crystals at ordinary temperature; yellow, orthorhombic crystals, stable above 489 °C
-
蒸汽压力:1 Pa at 724 °C; 10 Pa at 816 °C; 100 Pa at 928 °C; 1kPa at 1065 °C; 10 kPa at 1241 °C; 100 kPa at 1471 °C
-
稳定性/保质期:
-
如果遵照规格使用和储存,则不会分解,没有已知危险反应。避免氧化物或碱金属加热至300~450℃时变为四氧化三铅;继续升温又会变为一氧化铅。该物质不溶于水和乙醇,但能溶解于丙酮、硝酸、液碱及氯化铵中,并能与甘油发生硬化反应。有毒!
-
操作过程中,请佩戴口罩,以阻滞95%~97%的铅粉尘。当蒸汽浓度较高时,应使用过滤式防毒面具或软管式防毒面具,并强制供给新鲜空气。工作时间内禁止进食、吸烟。工作后需洗浴并漱口、刷牙;使用1%醋酸溶液清洗手部或其他受污染部位以去除铅残留。若出现铅中毒症状,应及时脱离岗位,并根据严重程度调整工作岗位。β-PbO为黄色正交晶系晶体,两种形式均不溶于水和乙醇,微溶于稀硝酸。在常温下研磨时,α-PbO会转变为β-PbO;而β-PbO向α-PbO转变的温度范围是475~583℃。
-
稳定
-
禁配物:强酸、强碱
-
聚合危害:不聚合
-
-
分解:When heated to decomposition it emits toxic fumes of /lead/.
计算性质
-
辛醇/水分配系数(LogP):-0.5
-
重原子数:2
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
ADMET
安全信息
-
TSCA:Yes
-
危险等级:6.1(b)
-
危险品标志:T,N
-
安全说明:S45,S53,S60,S61
-
危险类别码:R61,R33,R20/22,R50/53,R62
-
WGK Germany:3
-
海关编码:2824 10 00
-
危险品运输编号:UN 1479/2291
-
危险类别:6.1(b)
-
包装等级:III
制备方法与用途
一氧化铅,一般称为黄丹,可用于颜料、光学玻璃、陶瓷、橡胶、油漆催干剂、石油业、铅盐和粘合剂等行业。
毒性 参见一氧化铅。早期中毒症状包括齿龈边缘出现铅线(明显中毒时可能没有铅线)、皮肤呈土灰色。初期可能出现神经衰弱综合征,如果影响小脑,则可能导致铅中毒性抑郁症或躁狂症,并引发铅毒性麻痹和敏感性多发性神经炎。还会影响低色素贫血、代谢及内分泌障碍、消化系统等。
- 神经系统:抑制某些酶的活性,导致消化不良,腹部剧烈疼痛,肝脏受损。
- 内分泌系统:可能引起高血压和胆固醇增高。
如果出现腹痛,可进行皮下注射阿托品等药物,并采取腹部保暖、灌肠或洗热水浴等措施。空气中最大容许浓度为0.01 mg/m³。
操作时应戴上口罩以减少95%~97%的铅粉尘吸入;当蒸汽浓度过高时,则需使用过滤式防毒面具或软管式防毒面具,并确保新鲜空气供应。工作期间禁止进食和吸烟,结束后要洗淋浴、漱口和刷牙。手部或其他部位如有污染,应用1%醋酸溶液清洗。严重中毒者应暂时离开工作岗位。
化学性质 黄丹为黄色四方晶系粉末,不溶于水及乙醇,但可溶于丙酮、硝酸、液碱或氯化铵中。
用途 广泛用于冶炼金属铅、制铅玻璃和铅化合物、催化剂以及油漆催干剂;作为分析试剂、硅酸盐的助熔剂,也适用于氨基酸沉淀;在电子管、显像管、光学玻璃及防辐射橡胶制品制造中有应用价值;是聚氯乙烯塑料稳定剂的重要原料;还用于蓄电池极板制造与石油精制等。
生产方法 采用金属铅氧化法:将铅加热熔融制成铅粒,然后在170~210℃下进行磨粉处理,在600℃以上高温焙烧以实现氧化反应。 [ 2Pb + O_2 \rightarrow 2PbO ]
类别 有毒物品
毒性分级 高毒
急性毒性 腹腔注射-大鼠LDL0: 430 毫克/公斤;腹腔注射-小鼠LD50: 217 毫克/公斤
刺激数据 皮肤接触-兔子,100毫克/24小时为轻度刺激。
可燃性危险特性 不可燃物质;受热时产生有毒含铅化物烟雾。
储运特性 储存于通风干燥的库房中,并与食品添加剂分开存放。
职业标准 TLV-TWA 0.15 毫克/立方米;STEL 0.04 毫克/立方米。
上下游信息
反应信息
-
作为反应物:描述:氧化铅 以950%的产率得到参考文献:名称:PAVLOV, D.;MONAKHOV, B.;MAJA, M.;PENAZZI, N., J. ELECTROCHEM. SOC., 136,(1989) N, C. 27-33摘要:DOI:
-
作为产物:描述:碳酸铅(II) 以99%的产率得到参考文献:名称:NYITRAI, ZOLTAN;FILIP, LIVIU;ILLES, STEFAN;PASOI, NICOLAE;MAZUR, STEFAN摘要:DOI:
-
作为试剂:描述:一氧化碳 、 苯酚 在 氧化铅 、 bis(acetylacetonato)palladium(II) 、 四甲基氢氧化铵 、 四丁基硝酸铵 、 manganese(III) acetylacetonate 氧气 作用下, 100.0 ℃ 、11.0 MPa 条件下, 反应 3.0h, 生成 碳酸二苯酯参考文献:名称:Method and catalyst composition for producing aromatic carbonates摘要:本发明揭示了一种从芳香族羟基化合物经济生产芳香族碳酸酯的方法和催化剂组合物。本发明提供了一种羰基化芳香族羟基化合物的方法,包括将至少一种芳香族羟基化合物与氧气和一氧化碳接触,在无卤素羰基化催化剂组合物的存在下,所述无卤素羰基化催化剂组合物包括至少一种第8、9或10族金属源的有效量,至少一种第14族金属源的有效量的第一无机共催化剂,一种盐类共催化剂的有效量,以及可选的来自由第4族金属源、第7族金属源、第11族金属源和镧系元素源组成的羰基化催化剂组合物的第二无机共催化剂的有效量,以及可选的碱的有效量。本方法和催化剂组合物的一个重要优点是,在反应混合物中不含或不需要卤素以获得催化活性。公开号:US06700008B2
文献信息
-
Characterization of the chemiluminescence observed during the reaction between lead vapor and 3ΣO2作者:E.A. Dorko、J.W. Glessner、C.M. Ritchey、S.R. SnyderDOI:10.1016/0009-2614(84)85392-0日期:1984.8The chemiluminescence observed during the reaction between lead vapor and ground state oxygen (13ΣO 2) was analyzed. The spectral bands could be assigned to transitions from the a, A, B and C electronic states to the ground state. Evidence is presented to indicate that the reactive lead vapor is generated thermally rather than electrically.
-
Analysis of the chemiluminescence from electronically excited lead oxide generated in a flow tube reactor作者:E.A. Dorko、J.W. Glessner、C.M. Ritchey、L.L. Rutger、J.J. Pow、L.D. Brasure、J.P. Duray、S.R. SnyderDOI:10.1016/0301-0104(86)80007-6日期:1986.3The chemiluminescence from electronically excited lead oxide formed during the reaction between lead vapor and either 3Σ O2 or 1Δ O2 has been studied. The reactions were accomplished in a flow tube reactor. A microwave discharge was used to generate 1Δ O2. The vibronic spectrum was analyzed and the band head assignments were used in a linear least-squares calculation to obtain the vibronic molecular
-
Process for the production of basic soaps of divalent metals in powder申请人:Neynaber Chemie GmbH公开号:US04927548A1公开(公告)日:1990-05-22Powdered basic metal soaps having a composition corresponding to the formula (MO).sub.n.M(RCOO).sub.2, where MO represents CaO, ZnO, MgO, BaO, PbO, or a mixed oxide PbO/CaO, CaO/ZnO, BaO/CdO, or BaO/ZnO, M is one of the metals mentioned above, RCOO represents an anion of a fatty acid containing 8 to 34 carbon atoms, and n has a value of 0.2 to 2, may be obtained by reaction of powdered fatty acids with powdered metal oxides in the presence of small amounts of water, alcohol, and/or an acid at temperatures between ambient temperature and 100.degree. C. and under a pressure not greater than ambient. Preferably, there is continuous removal of water formed during the reaction, and the reaction mixture is present as free flowing particles throughout the reaction.
-
Method for the preparation of IV-VI semiconductor nanoparticles申请人:Cho Kyung-Sang公开号:US20060110313A1公开(公告)日:2006-05-25A high temperature (on the order of about 90° C. or above) non-aqueous synthetic procedure for the preparation of substantially monodisperse IV-VI semiconductor nanoparticles (quantum dots) is provided. The procedure includes first introducing a first precursor selected from the group consisting of a molecular precursor of a Group IV element and a molecular precursor of a Group VI element into a reaction vessel that comprises at least an organic solvent to form a mixture. Next, the mixture is heated to a temperature of about 90° C. or above and thereafter a second precursor which is different from the first precursor and is selected from the group consisting of a molecular precursor of a Group IV element and a molecular precursor of a Group VI element is added into the heated mixture. The reaction mixture is then mixed to initiate nucleation of IV-VI nanocrystals and the temperature of the reaction mixture is controlled to provide substantially monodispersed IV-VI nanoparticles having a diameter of about 20 nm or less.提供了一种高温(大约90°C或以上)的非水合成方法,用于制备基本单分散的IV-VI半导体纳米颗粒(量子点)。该方法首先将选自Group IV元素的分子前体或Group VI元素的分子前体之一的第一前体引入至至少包含有机溶剂的反应容器中形成混合物。接下来,将混合物加热至大约90°C或以上,然后向加热的混合物中加入与第一前体不同的第二前体,所述第二前体选自Group IV元素的分子前体或Group VI元素的分子前体之一。然后混合反应物以启动IV-VI纳米晶的成核,并控制反应物的温度,以提供直径约为20nm或更小的基本单分散的IV-VI纳米颗粒。
-
[1-Oxo-2-thienyl-2-substituted-5-indanyloxy (or thio)]alkanoic acids and申请人:Merck & Co., Inc.公开号:US04177285A1公开(公告)日:1979-12-04[1-Oxo-2-aryl or thienyl-2-substituted-5-indanyloxy (or thio)]alkaoic acid, derivatives thereof, their salts, esters and amides are disclosed. The products display a dual pharmaceutical utility in that they exhibit diuretic, saluretic and uricosuric activity. Also disclosed are processes for the preparation of such [1-oxo-2-aryl or thienyl-2-substituted-5-indanyloxy (or thio)]alkanoic acids, pharmaceutical compositions comprising therapeutically effective amounts of such compounds and methods of treatment comprising administering such compounds and compositions.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
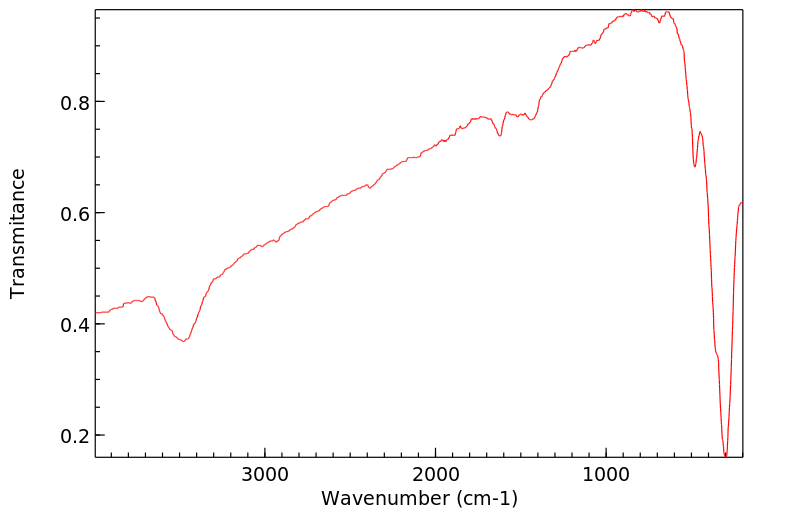
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息