1-[(E)-1,3-Thiazol-2-yldiazenyl]-2-naphthol | 61433-07-6
中文名称
——
中文别名
——
英文名称
1-[(E)-1,3-Thiazol-2-yldiazenyl]-2-naphthol
英文别名
1-(2-thiazolylazo)-2-naphthalenol;1-(2-thiazolylazo)-2-naphthol;1-(Thiazol-2-ylazo)-<2>naphthol;1-(2-Thiazolylazo)-2-naphtol;1-trans-thiazol-2-ylazo-naphthalen-2-ol;[1,2]naphthoquinone 1-((Z)-thiazol-2-yl-hydrazone);1-(thiazol-2-yl-trans-azo)-[2]naphthol
CAS
61433-07-6
化学式
C13H9N3OS
mdl
——
分子量
255.3
InChiKey
IOMXCGDXEUDZAK-FOCLMDBBSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.42
-
重原子数:18.0
-
可旋转键数:2.0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:57.84
-
氢给体数:1.0
-
氢受体数:5.0
反应信息
-
作为反应物:参考文献:名称:GORELIK, M. V.;RYBINOV, V. I.;GLADYSHEVA, T. X., ZH. OBSHCH. XIMII, 58,(1988) N0, S. 2360-2370摘要:DOI:
-
作为产物:参考文献:名称:GORELIK, M. V.;RYBINOV, V. I.;GLADYSHEVA, T. X., ZH. OBSHCH. XIMII, 58,(1988) N0, S. 2360-2370摘要:DOI:
文献信息
-
Potentiometric, spectroscopic and thermal studies on the metal chelates of 1-(2-thiazolylazo)-2-naphthalenol作者:M.M. Omar、Gehad G. MohamedDOI:10.1016/j.saa.2004.05.040日期:2005.3[M(L)2] for M = Mn(II), Co(II), Ni(II), Zn(II) and Cd(II); [M(L)X].nH2O for M = Cu(II) (X = AcO, n = 3), Pd(II) (X = Cl, n = 0) and UO2(II) (X = NO3, n = 0), and [Fe(L)Cl2(H2O)].2H2O. The molar conductance data reveal that the chelates are non-electrolytes. IR spectra show that the ligand is coordinated to the metal ions in a terdentate manner with ONN donor sites of the naphthyl OH, azo N and thiazoleMn(II),Fe(III),Co(II),Ni(II),Cu(II),Zn(II),Cd(II),Pd(II)和UO2(II)螯合物的合成与表征据报道1-(2-噻唑基偶氮)-2-萘酚(TAN)。配体的解离常数和金属络合物的稳定性常数是在25摄氏度和离子强度为0.1 M的条件下,通过pH值计算得出的。通过元素和热分析,摩尔电导,IR,磁和漫反射光谱对固体配合物进行表征。对于M = Mn(II),Co(II),Ni(II),Zn(II)和Cd(II),发现配合物具有式[M(L)2]。[M(L)X] .n ,用于M = Cu(II)(X = AcO,n = 3),Pd(II)(X = Cl,n = 0)和UO2(II)(X = NO3,n = 0)和[Fe(L)Cl2(H2O)]。2 。摩尔电导数据表明,螯合物是非电解质。红外光谱表明,配体与萘,OH,偶氮和噻唑N的ONN供体位点呈齿状
-
AIR BAG GAS GENERATING AGENT申请人:OTSUKA KAGAKU KABUSHIKI KAISHA公开号:EP0763512A1公开(公告)日:1997-03-19An object of this invention is to provide an air bag azide-free gas generating composition which can pronouncedly reduce the concentrations of toxic components in the after gas, especially both concentrations of CO and NOx while retaining the desired advantages of azide-free gas generating compositions. The air bag gas generating composition of the invention is an azide-free gas generating composition comprising a nitrogen-containing organic compound and an oxidizing agent as active components, and further contains, as a burning catalyst, at least one metallic oxide selected from the group consisting of molybdenum oxides, tungsten oxides and metallic oxides having a BET specific surface area of at least 5 m2/g.
-
Neues Verfahren zur Herstellung von 3-Stellung funktionalisierten Organosilanen申请人:Degussa-Hüls Aktiengesellschaft公开号:EP0963993A2公开(公告)日:1999-12-15Die vorliegende Erfindung betrifft ein neues Verfahren zur Herstellung von in 3-Stellung funktionalisierten Organosilanen durch die Umsetzung von geeigneten Allylverbindungen mit Hydrogensilanen unter Verwendung von Platinkatalysatoren, die einen oder mehrere schwefelhaltige Liganden besitzen.
-
OPTICAL RECORDING MEDIUM AND METHOD OF PRODUCING THE SAME申请人:MATSUSHITA ELECTRIC INDUSTRIAL CO., LTD.公开号:EP0995612A1公开(公告)日:2000-04-26A write-once optical recording medium having on a substrate a recording layer on which information can be written and/or read out therefrom with a laser beam, where a proper tracking error signals is ensured over the whole substrate of the medium. The medium is adaptable to high information recording density. This medium is produced by forming a recording layer by vacuum depositing on the substrate a recording layer-constituting material containing a metal-containing azo compound having a weight-loss starting temperature or a decomposition temperature ranging from 300 to 500 °C.一种一次性写入光学记录介质,其基板上有一个记录层,可以用激光束在上面写入和/或读出信息,在介质的整个基板上确保有适当的跟踪误差信号。这种介质可适应高信息记录密度。这种介质是通过在基底上真空沉积含有含金属偶氮化合物的记录层构成材料来形成记录层的,这种材料的失重起始温度或分解温度在 300 ℃ 至 500 ℃ 之间。
-
Liver cell clones for artifical liver and extracorporeal liver assist device申请人:JMS Co., Ltd.公开号:EP1063289A1公开(公告)日:2000-12-27Disclosed is a blood perfusion device or apparatus that is used for bioartificial liver support. Human hepatocyte lines established from normal regenerating liver tissue and modulated in toxin-challenging conditions are provided. These functional hepatocytes exhibit extraordinarily enhanced detoxification functions, which are characterised by the elevated glutathione content and glutathione S-transferase activity. A bioreactor is constructed with the functional hepatocytes for bioartificial liver support system, which includes perfusion inlets and perfusion outlets, a containment vessel, a centrifugal pump and macroporous microcarriers where the functional hepatocytes are grown.
表征谱图
-
氢谱1HNMR
-
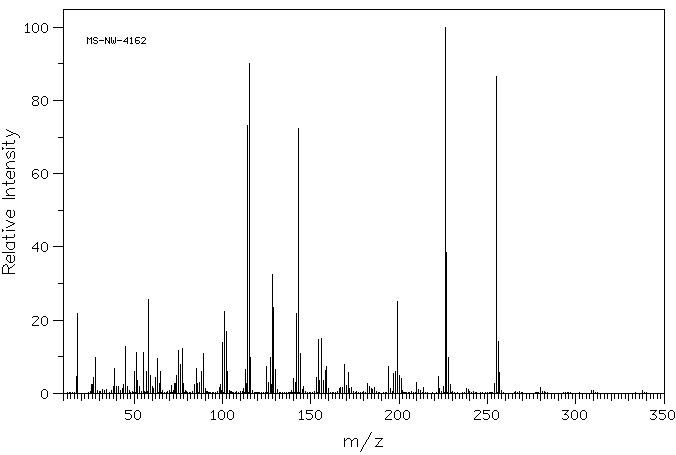
质谱MS
-
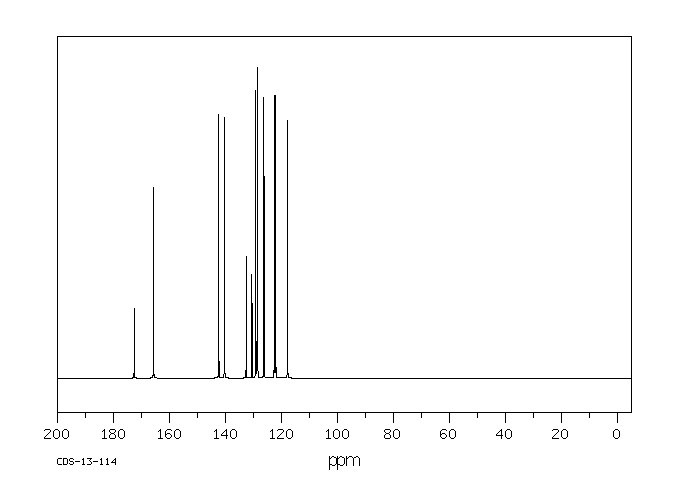
碳谱13CNMR
-
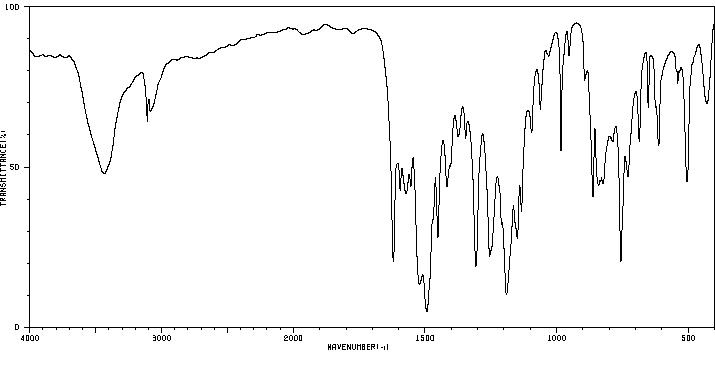
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮