rel-1alpha*-氟-2beta*-溴环己烷 | 17170-96-6
中文名称
rel-1alpha*-氟-2beta*-溴环己烷
中文别名
——
英文名称
trans-1-bromo-2-fluorocyclohexane
英文别名
1-bromo-2-fluoro-cyclohexane;(1R,2R)-1-bromo-2-fluorocyclohexane
CAS
17170-96-6
化学式
C6H10BrF
mdl
——
分子量
181.048
InChiKey
AZQRVGXSORXOCR-PHDIDXHHSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:30 °C(Press: 13 Torr)
-
密度:1.40±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2903890090
SDS
反应信息
-
作为反应物:描述:参考文献:名称:顺式和反式-1-溴-2-氟环己烷的脱卤化氢反应摘要:研究了两种非对映体邻位溴氟环己烷与苏打酰胺,甲醇钠,叔丁醇钾和三乙胺的反应。顺式-1-溴-2-氟环己烷几乎完全消除了溴化氢,主要产生了1-氟环己烯和少量的3-氟环己烯。反式-1-溴-2-氟环己烷在用酰胺溶液处理时优先消除了氟化氢。钾叔-丁醇钾消除溴化氢,并且得到3- fluorocyclohexene和1,3-环己二烯的量小,而甲醇钠转化反-1-溴-2-氟环己烷为3-氟环己烯和3-甲氧基环己烯。讨论了优先消除氟化氢的可能机理和立体化学,并提供了新的解释。DOI:10.1016/s0022-1139(00)81950-8
-
作为产物:描述:环己烯 在 N-溴代丁二酰亚胺(NBS) 、 3-ethyl-1-methylimidazolium oligo hydrogen fluoride 作用下, 以 二氯甲烷 为溶剂, 反应 1.0h, 以90%的产率得到rel-1alpha*-氟-2beta*-溴环己烷参考文献:名称:用离子液体EMIMF(HF)2.3作为温和的HF源进行氟化摘要:氟化氢是一种基本的氟化试剂,但是处理起来很困难。为此,已经开发了一些改性的氟化试剂,例如HF-吡啶,Et 3 N-HF和聚(氟化氢)络合物。但是,这些试剂仍需要进行水溶液后处理程序,以产生氟化氢。近来,由于易于处理和非水后处理的可能性,离子液体受到了广泛的关注。在空气和湿气中稳定的离子液体3-乙基-1-甲基咪唑鎓低聚氟化氢(EMIMF(HF)2.3)可以用作某些氟化反应的氟化氢当量;它不需要水后处理。DOI:10.1016/j.jfluchem.2005.09.016
文献信息
-
Selective Halofluorination of Alkenes with Tetrabutylphosphonium Dihydrogentrifluoride in Combination with<i>N</i>-Halosuccinimide or 1,3-Dibromo-5,5-dimethylhydantoin作者:Yukitaka Uchibori、Masayuki Umeno、Hideharu Seto、Hirosuke YoshiokaDOI:10.1246/cl.1993.673日期:1993.4Alkenes and their functionalized derivatives were readily converted to the corresponding halofluorides with tetrabutylphosphonium dihydrogentrifluoride as combined with N-halosuccinimides or 1,3-dibromo-5,5-dimethylhydantoin in highly regio-, stereo-, and chemoselective manners. In particular, alkenes having a oxirane or primary hydroxyl group also underwent halofluorination selectively in good yields
-
Potassium Fluoride-Poly(Hydrogen Fluoride) Salts as Fluorinating Agents for Halofluorination of Alkenes作者:Masanori Tamura、Motonari Shibakami、Akira SekiyaDOI:10.1055/s-1995-3946日期:1995.5It was found that potassium fluoride-poly(hydrogen fluoride) salts are useful fluorine sources for halofluorination of alkenes. The reaction proceeded with these salts and N-halosuccinimides or 1,3-dibromo-5,5-dimethylhydantoin in a regio- and stereoselective manner.
-
Dispersion and Halogen-Bonding Interactions: Binding of the Axial Conformers of Monohalo- and (±)-<i>trans</i>-1,2-Dihalocyclohexanes in Enantiopure Alleno-Acetylenic Cages作者:Cornelius Gropp、Tamara Husch、Nils Trapp、Markus Reiher、François DiederichDOI:10.1021/jacs.7b05461日期:2017.9.6means to study the elusive axial/diaxial conformers. X-ray co-crystal structures of AACs further allowed for a detailed investigation, both experimentally and theoretically, on the interplay between space occupancy, guest conformation, and chiral recognition based purely on dispersion forces and weak C-X···π (X = Cl, Br, I) and C-X···||| (acetylene) contacts (X = Cl, Br). The theoretical analysis of具有间苯二酚 [4] 芳烃支架的对映体纯丙炔-炔笼 (AAC) 受体,四个同手性丙炔-乙炔从中汇聚形成一个由四重 OH-氢键阵列封闭的空腔,在其中形成高度有序的多孔网络固态。它们能够使原本非结晶的小分子络合和共结晶。本文分析了结合在 AAC 内腔中的单卤和 (±)-反式 1,2-二卤代环己烷的轴向构象异构体,在固态和溶液中的原子水平上,并伴随着准确的计算。轴向/双轴构象异构体的二面角 ϑa,a (XC(1)-C(2)-X/H) 从 180° 大幅偏离,下降到 144°,伴随着环二面角的强烈扁平化。孤立客体分子的结构优化表明,与主体的非共价相互作用几乎不影响二面角,证实主体是研究难以捉摸的轴向/二轴构象异构体的理想手段。AAC 的 X 射线共晶结构进一步允许从实验和理论上对空间占有、客体构象和手性识别之间的相互作用进行详细研究,这些相互作用仅基于色散力和弱 CX…π (X = Cl , Br
-
Poly-4-vinylpyridinium Poly(Hydrogen Fluoride): A Solid Hydrogen Fluoride Equivalent Reagent作者:George A. Olah、Xing-Ya Li、Qi Wang、G. K. Surya PrakashDOI:10.1055/s-1993-25924日期:——Poly-4-vinylpyridinium poly(hydrogen fluoride) (PVPHF), containing 35-60% hydrogen fluoride by weight, was prepared as a solid hydrogen fluoride equivalent reagent. PVPHF with 60% hydrogen fluoride by weight was found to be a versatile fluorinating agent for the hydrofluorination and bromofluorination of alkenes and alkynes, fluorination of alcohols as well as other fluorination reactions. Low hydrogen fluoride content PVPHF (3 equivalents of hydrogen fluoride to 1 equivalent of 4-vinylpyridine unit) was also found to be an efficient reagent for bromofluorination of alkenes in the presence of 1,3-dibromo-5,5-dimethylhydantoin. Fluorosulfonic acid-modified PVPHF showed enhanced reactivities for the fluorination of secondary alcohols.
-
Synthesis of sp3-Enriched β-Fluoro Sulfonyl Chlorides作者:Oleksandr O. Grygorenko、Rustam Gurbanov、Andriy Sokolov、Sergey Golovach、Kostiantyn Melnykov、Alexey V. DobrydnevDOI:10.1055/s-0040-1706101日期:2021.5A three-step approach to the synthesis of sp3-enriched β-fluoro sulfonyl chlorides starting from alkenes is reported. The method was successfully applied to a wide range of acyclic and cyclic substrates, bearing either an exocyclic or an endocyclic double bond. The procedure worked with a wide range of substrates and tolerated a number of functional and protecting groups. Moreover, the target cyclic
表征谱图
-
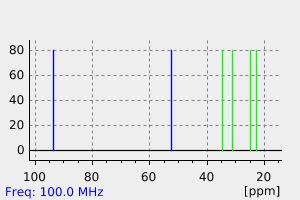
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-2-氯环己基高氯酸盐
顺式-1-溴-2-氟-环己烷
顺式-1-叔丁基-4-氯环己烷
顺式-1,2-二氯环己烷
顺-1H,4H-十二氟环庚烷
镓,三(三氟甲基)-
镁二(1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8,8-十七氟-1-辛烷磺酸酯)
铵2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,12-二十三氟十二烷酸盐
铜N-(2-氨基乙基)乙烷-1,2-二胺2-氰基胍二氯化盐酸
钾{[(十七氟辛基)磺酰基](甲基)氨基}乙酸酯
钠3-[(3-{[(十七氟辛基)磺酰基]氨基}丙基)(甲基)氨基]-1-丙烷磺酸酯
重氮基烯,(1-溴环己基)(1,1-二甲基乙基)-,1-氧化
辛酸,十五氟-,2-(1-羰基辛基)酰肼
赖氨酰-精氨酰-精氨酰-苯基丙氨酰-赖氨酰-赖氨酸
诱蝇羧酯B1
诱蝇羧酯
萘并[2,1-b]噻吩-1(2H)-酮
膦基硫杂酰胺,P,P-二(三氟甲基)-
脲,N-(4,5-二甲基-4H-吡唑-3-基)-
肼,(3-环戊基丙基)-,盐酸(1:1)
组织蛋白酶R
磷亚胺三氯化,(三氯甲基)-
碳标记全氟辛酸
碘甲烷与1-氮杂双环(4.2.0)辛烷高聚合物的化合物
碘甲烷-d2
碘甲烷-d1
碘甲烷-13C,d3
碘甲烷
碘环己烷
碘仿-d
碘仿
碘乙烷-D1
碘[三(三氟甲基)]锗烷
硫氰酸三氯甲基酯
甲烷,三氯氟-,水合物
甲次磺酰胺,N,N-二乙基-1,1,1-三氟-
甲次磺酰氯,氯二[(三氟甲基)硫代]-
甲基碘-12C
甲基溴-D1
甲基十一氟环己烷
甲基丙烯酸正乙基全氟辛烷磺
甲基三(三氟甲基)锗烷
甲基[二(三氟甲基)]磷烷
甲基1-氟环己甲酸酯
环戊-1-烯-1-基全氟丁烷-1-磺酸酯
环己烷甲酸4,4-二氟-1-羟基乙酯
环己烷,1-氟-2-碘-1-甲基-,(1R,2R)-rel-
环己基五氟丙烷酸酯
环己基(1-氟环己基)甲酮
烯丙基十七氟壬酸酯