二正壬基胺 | 2044-21-5
中文名称
二正壬基胺
中文别名
二壬基胺
英文名称
di(n-nonyl)amine
英文别名
dinonylamine;N-nonyl-nonan-1-amine;N-nonylnonan-1-amine
CAS
2044-21-5
化学式
C18H39N
mdl
MFCD00027338
分子量
269.514
InChiKey
MFHKEJIIHDNPQE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:24°C
-
沸点:336°C
-
密度:0.8055
-
闪点:24 °C
-
稳定性/保质期:
如果遵照规格使用和储存,则不会分解,未有已知危险反应。请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):7.7
-
重原子数:19
-
可旋转键数:16
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:12
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:8
-
安全说明:S26,S36/37/39
-
危险类别码:R34
-
海关编码:2921199090
-
危险品运输编号:UN 2735
-
储存条件:请将贮藏器密封,并将其存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
Dinonylamine Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Dinonylamine
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 1C
Category 1
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements Causes severe skin burns and eye damage
Precautionary statements:
[Prevention] Do not breathe dust/fume/gas/mist/vapours/spray.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
[Response]
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Dinonylamine
Percent: >97.0%(GC)
2044-21-5
CAS Number:
Chemical Formula: C18H39N
Dinonylamine
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (P3 filter respirator for toxic particles). Keep
protective equipment and people away from and upwind of spill/leak. Entry to non-involved personnel should
emergency procedures: be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a closed system if possible. Use a local exhaust if dust or aerosol will be
generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Protect from moisture.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Hygroscopic
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Dinonylamine
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Respiratory protection: Dust respirator, self-contained breathing apparatus(SCBA), supplied air respirator,
etc. Use respirators approved under appropriate government standards and follow
local and national regulations.
Hand protection: Impervious gloves.
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: Crystal
Colour: White - Almost white
Odour: No data available
pH: No data available
Melting point/freezing point:24°C (Freezing point)
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Dinonylamine
Section 12. ECOLOGICAL INFORMATION
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 8: Corrosive.
3259
UN-No:
Proper shipping name: Amines, solid, corrosive, n.o.s.
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Dinonylamine
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 1C
Category 1
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Danger
Hazard statements Causes severe skin burns and eye damage
Precautionary statements:
[Prevention] Do not breathe dust/fume/gas/mist/vapours/spray.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
[Response]
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Dinonylamine
Percent: >97.0%(GC)
2044-21-5
CAS Number:
Chemical Formula: C18H39N
Dinonylamine
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (P3 filter respirator for toxic particles). Keep
protective equipment and people away from and upwind of spill/leak. Entry to non-involved personnel should
emergency procedures: be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a closed system if possible. Use a local exhaust if dust or aerosol will be
generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store under inert gas.
Protect from moisture.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Hygroscopic
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Dinonylamine
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Respiratory protection: Dust respirator, self-contained breathing apparatus(SCBA), supplied air respirator,
etc. Use respirators approved under appropriate government standards and follow
local and national regulations.
Hand protection: Impervious gloves.
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: Crystal
Colour: White - Almost white
Odour: No data available
pH: No data available
Melting point/freezing point:24°C (Freezing point)
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx)
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Dinonylamine
Section 12. ECOLOGICAL INFORMATION
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 8: Corrosive.
3259
UN-No:
Proper shipping name: Amines, solid, corrosive, n.o.s.
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:参考文献:名称:Synthesis and antimicrobial activity of symmetrical two-tailed dendritic tricarboxylato amphiphiles摘要:Two series of water-soluble, symmetrical two-tailed homologous dendritic amphiphiles-R2NCONHC((CH2)(2)COOH)(3), 2(n,n), and R2CHNHCONHC((CH2))(2)COOH)(3), 3(n,n), where R = n-CnH2n+1-were synthesized and compared to R"NHCONHC((CH2)(2)CoOH)(3), 1(n), R" = n-CnH2n+1, to determine whether antimicrobial activity was influenced by total or individual alkyl chain lengths, and whether antimicrobial activity depends on hydrophobicity or tail topology (one or two). In a broad screen of 11 microorganisms, 2(n,n) and 3(n,n) generally displayed higher minimal inhibitory concentrations (MICs) than I(n) against growth as measured by broth microdilution assays. Chain-length specificity was observed against Candida albicans as 1(16), 2(8,8), and 3(8,8) showed the lowest MIC in their respective series. The one case where two-tailed compounds displayed the lowest MICs-3(10,10), 15 mu M; 3(11,11), 7.2 mu M; and 3(12,12), 6.9 mu M-was against Cryptococcus neoformans. (c) 2007 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2007.03.017
-
作为产物:参考文献:名称:Mitsunobu synthesis of symmetrical alkyl and polyfluoroalkyl secondary amines摘要:Trifluoromethanesulfonamides (triflamides) having the structure CF3SO2N[(CH2)(3)R](2) (R = CnF2n+1 or CnH2n+1, n = 4, 6, 8, 10) are obtained in high yields, when CF3SO2NH2 is reacted with 3-perfluoroalkyl-1-propanols or the parent aliphatic alcohols in a Mitsunobu reaction (Ph3P/[i-PrO2CN=NCO2-i-Pr]/ether). Products are isolated easily by fluorous extraction, fluorous solid-organic liquid filtration or n-heptane/CH3OH extraction. Consecutive deprotection of triflamides with LiAlH4 in boiling ether or dioxane solution affords the title amines in good overall yields. Fluorous partition coefficients of the F-tagged amides and amines are determined and qualitatively analyzed. (c) 2005 Elsevier B.V. All rights reserved.DOI:10.1016/j.jfluchem.2005.08.012
-
作为试剂:参考文献:名称:Production of primary amines摘要:公开号:US02408959A1
文献信息
-
A modular approach for multicomponent synthesis of amidines using modified Scolecite作者:Megha Jagadale、Pallavi Bhange、Rajashri Salunkhe、Deu Bhange、Mohan Rajmane、Gajanan RashinkarDOI:10.1016/j.apcata.2015.11.010日期:2016.2Copper exchanged Scolecite was successfully synthesized and used as an efficient, mild and recyclable catalyst for promoting multi-component synthesis of amidines from 4-toluenesulfonyl azide, phenyl acetylene and variety of substituted amines in THF at ambient temperature in good yields within short reaction time.
-
[EN] UREA DERIVATIVES OF AMPHOTERICIN B DERIVED FROM SECONDARY AMINES<br/>[FR] DÉRIVÉS D'URÉE DE L'AMPHOTÉRICINE B DÉRIVÉE D'AMINES SECONDAIRES申请人:UNIV ILLINOIS公开号:WO2016112243A1公开(公告)日:2016-07-14Provided are certain urea derivatives of amphotericin B (AmB) having improved therapeutic index compared to AmB. The compounds of the invention are less toxic than AmB and are useful to treat fungal infections. In certain embodiments the urea derivative of AmB is a compound represented by formula (I) or a pharmaceutically acceptable salt thereof: wherein, independently for each occurrence: R represents methyl, ethyl, propyl, or isopropyl; R' represents methyl, ethyl, propyl, or isopropyl; or R and R', taken together with the nitrogen atom to which they are attached, represent a radical of a cyclic secondary amine. Also provided are methods for making the urea derivatives of AmB.
-
Mononuclear iron complex and organic synthesis reaction using same申请人:KYUSHU UNIVERSITY, NATIONAL UNIVERSITY CORPORATION公开号:US10363551B2公开(公告)日:2019-07-30A mononuclear iron bivalent complex having iron-silicon bonds, which is represented by formula (1), can exhibit an excellent catalytic activity in at least one reaction selected from three reactions, i.e., a hydrosilylation reaction, a hydrogenation reaction and a reaction for reducing a carbonyl compound. (In the formula, R1 to R6 independently represent a hydrogen atom, an alkyl group which may be substituted by X, or the like; X represents a halogen atom, or the like; L1 represents at least one two-electron ligand selected from an isonitrile ligand, an amine ligand, an imine ligand, a nitrogenated heterocyclic ring, a phosphine ligand, a phosphite ligand and a sulfide ligand, wherein, when multiple L1's are present, two L1's may be bonded to each other; L2 represents a two-electron ligand that is different from a CO ligand or the above-mentioned L1, wherein, when multiple L2's are present, two L2's may be bonded to each other; and m1 represents an integer of 1 to 4 and m2 represents an integer of 0 to 3, wherein the sum total of m1 and m2 (i.e., m1+m2) satisfies 3 or 4.)
-
Salt suitable for an acid generator and a chemically amplified resist composition containing the same申请人:Harada Yukako公开号:US20070078269A1公开(公告)日:2007-04-05The present invention provides a salt of the formula (I): wherein ring Y represents monocyclic or polycyclic hydrocarbon group having 3 to 30 carbon atoms, in which one —CH 2 — group is substituted with —COO— group, and at least one hydrogen atom in the monocyclic or polycyclic hydrocarbon group may optionally be substituted with alkyl group having 1 to 6 carbon atom, alkoxy group having 1 to 6 carbon atom, perfluoroalkyl group having 1 to 4 carbon atoms, hydroxyalkyl group having 1 to 6 carbon atoms, hydroxyl group or cyano group; Q 1 and Q 2 each independently represent fluorine atom or perfluoroalkyl group having 1 to 6 carbon atoms; A + represents organic counter ion; and n shows an integer of 0 to 12. The present invention also provides a chemically amplified resist composition comprising the salt of the formula (I).
-
SALT AND PHOTORESIST COMPOSITION CONTAINING THE SAME申请人:ICHIKAWA Koji公开号:US20100304293A1公开(公告)日:2010-12-02A salt represented by the formula (a): wherein Q 1 and Q 2 each independently represent a fluorine atom etc., X 1 represents a single bond etc., X 2 represents a single bond etc., Y 1 represents a C3-C6 alicyclic hydrocarbon group etc., with the proviso that —X 2 —Y 1 group has one or more fluorine atoms, and Z + represents an organic counter cation, and a photoresist composition comprising the salt represented by the formula (a) and a resin comprising a structural unit having an acid-labile group and being insoluble or poorly soluble in an aqueous alkali solution but becoming soluble in an aqueous alkali solution by the action of an acid.
表征谱图
-
氢谱1HNMR
-
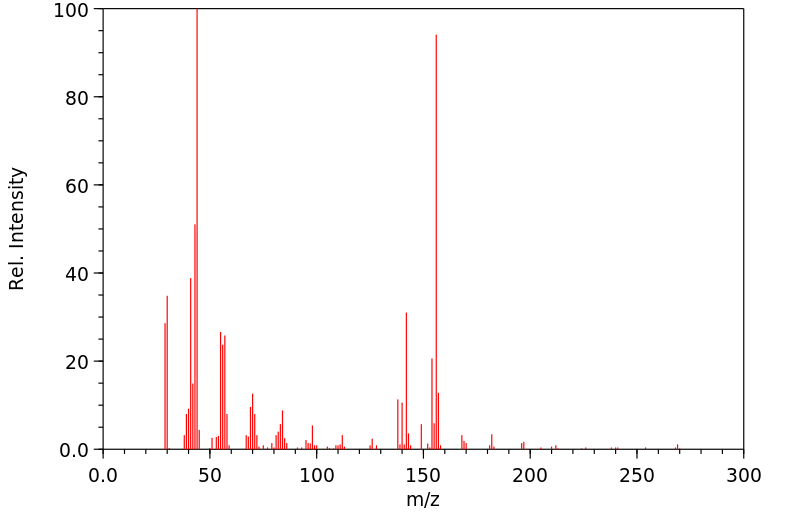
质谱MS
-
碳谱13CNMR
-
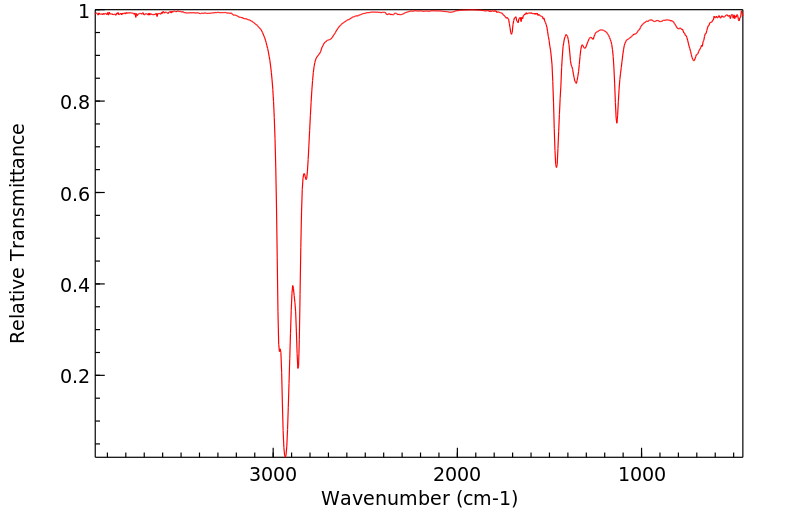
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷