二氧化锗 | 1310-53-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:>400 °C(lit.)
-
密度:6.239 g/mL at 25 °C
-
溶解度:乙二醇:可溶
-
LogP:b/nm
-
物理描述:DryPowder
-
颜色/状态:COLORLESS, HEXAGONAL CRYSTALS
-
折光率:INDEX OF REFRACTION: 1.650
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,也未发现有已知的危险反应,请避免接触氯化氢。
计算性质
-
辛醇/水分配系数(LogP):-0.62
-
重原子数:3
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
TSCA:Yes
-
危险品标志:Xn
-
安全说明:S22,S24/25
-
危险类别码:R22
-
WGK Germany:1
-
海关编码:2825600001
-
危险品运输编号:UN 1418
-
RTECS号:LY5240000
-
危险标志:GHS07
-
危险性描述:H302,H332
-
储存条件:应存放在通风干燥的库房中,并将包装密封以防水分。避免与碱类和酸类接触。在搬运过程中要注意轻拿轻放,以防玻璃瓶破损。
SDS
模块 1. 化学品
1.1 产品标识符
: 氧化锗(IV)
产品名称
1.2 鉴别的其他方法
Germanium dioxide
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性毒性, 经口 (类别 4)
急性毒性, 吸入 (类别 4)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H302 吞咽有害。
H332 吸入有害。
警告申明
预防
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P271 只能在室外或通风良好之处使用。
响应
P301 + P312 如果吞下去了:
如感觉不适,呼救解毒中心或看医生。如吞咽:如感觉不适,呼叫解毒中
心或就医。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P312 如感觉不适,呼救中毒控制中心或医生.
P330 漱口。
处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: Germanium dioxide
别名
: GeO2
分子式
: 104.64 g/mol
分子量
组分 浓度或浓度范围
Germanium dioxide
-
化学文摘登记号(CAS 1310-53-8
No.) 215-180-8
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
长期或频繁接触会导致:, 可能发生对肾的伤害。, 可能发生对肝的伤害。, 血液病, 电解质失衡, 神经毒性,
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
氧化锗
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
联合国运输名称: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
联合国运输名称: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粉末
颜色: 白色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: > 400 °C - lit.
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
不适用
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
3.637 g/cm3 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 大鼠 - 1,250 mg/kg
半数致死浓度(LC50) 吸入 - 大鼠 - 4 h - > 1,420 mg/m3
备注: 肺,胸,或者呼吸系统:呼吸困难 皮肤及附属物:其他:头发。
营养与总代谢:体重降低或体重增长减小。
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
生殖毒性 - 大鼠 - 未报道的
对新生儿的影响:身体的。
发育毒性 - 大鼠 - 未报道的
特定发育异常:肌肉骨骼系统。
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入有害。 可能引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
长期或频繁接触会导致:, 可能发生对肾的伤害。, 可能发生对肝的伤害。, 血液病, 电解质失衡, 神经毒性,
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: LY5240000
模块 12. 生态学资料
12.1 生态毒性
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
二氧化锗(化学式:GeO₂)的分子量为104.59,主要存在不溶性和可溶性两种变体。不溶性变体呈无色四方晶系棱柱体状,熔点约为1086℃,相对密度为6.239,折光率为1.99。它不溶于水和酸、碱溶液中,仅在550℃下与10倍量的NaOH共融。可溶性变体则为无色六方晶系菱形晶体,熔点约为1115℃,相对密度为4.228,折光率为1.695。这种形式易溶于水,并能与酸、碱溶液反应。
这两种变体在约1033℃下会发生转变,且这一过程较为缓慢。此外,六方晶系的二氧化锗在骤冷后可转化为无色透明玻璃状体,该状态下的物质容易溶解于盐酸生成四氯化锗或溶于氢氟酸生成六氟合锗酸;四方晶系的二氧化锗化学性质相对惰性,仅能与过量熔碱及熔碳酸钠反应。
制备方法由四氯化锗水解可制得二氧化锗。具体步骤为:在四氯化锗中加入6.5倍体积的蒸馏水并搅拌,静置一昼夜后生成二氧化锗沉淀,用冷水洗涤至洗液不含Cl⁻为止,并于200℃干燥得到产品。
毒性干燥的氧化锗对皮肤无刺激作用,但对结膜有轻微刺激。空气中最高容许浓度为2mg/m³。操作时应注意防止锗和氧化锗气溶胶对人体呼吸器官及皮肤的影响,务必佩戴适当的防护装备。
化学性质二氧化锗为白色粉末或六方晶系、四方晶系的固体形式。六方晶系中相对密度约为4.228,熔点约为1115℃;四方晶系则为6.239和约1086℃。不溶于水及盐酸,但能与碱溶液反应生成锗酸盐。
用途二氧化锗广泛应用于制备高纯度金属锗、特种玻璃、磷光材料以及晶体管等半导体材料的中间体。此外,也可用于电子工业中的多种产品制造,如取锗锭、有机锗、锗酸铋晶体、四氯化锗(用于光纤)及荧光粉和锗玻璃原料等。
生产方法采用四氯化锗水解法制备二氧化锗:在四氯化锗中加入6.5倍体积的蒸馏水搅拌后静置一昼夜,生成沉淀并用水洗至不含Cl⁻离子为止,在200℃下干燥即得产品。
反应信息
-
作为反应物:参考文献:名称:HARADA, ISAO;YODA, YUKIHIRO;IWANAGA, NARUYUKI;NISHITSUJI, TOSHIHIKO;KIKKA+摘要:DOI:
-
作为产物:参考文献:名称:CHERNICHENKO, V. A.;MINYAJLO, YU. G.;ZADORSKIJ, V. M.摘要:DOI:
文献信息
-
Interaction of simple amino acids (glycine, α-alanine, β-alanine and L-valine) with germatranol hydrate作者:Igor S. Ignatyev、Denis V. Lezov、Yulia A. Kondratenko、Tatyana A. KochinaDOI:10.1016/j.molstruc.2021.132245日期:2022.4[HOGeN(CH2CH2O)3]H2O with simple amino acids (glycine, α-alanine, β-alanine and L-valine) and characterized by the IR and 1H, 13C NMR spectroscopy. Equilibrium structures of corresponding molecules were obtained by the ωB97X-D DFT and B3LYP/ 6–31+G(d) methods. For molecules with amino residues H2NCHR-COO− (RH, Me, i-Pr), i.e. germatranyl glycinate (I), α-alaninate (II), and L-valinate (IV) two low-lying氨基羧基锗烷是通过水合锗烷醇 [HOGeN(CH 2 CH 2 O) 3 ]H 2 O 与简单氨基酸(甘氨酸、α-丙氨酸、β-丙氨酸和 L-缬氨酸)反应合成的,并通过 IR 和1 H表征, 13 C NMR 光谱。通过ωB97X-D DFT和B3LYP/ 6-31+ G (d)方法获得相应分子的平衡结构。对于具有氨基残基 H 2 N CHR-COO − (R H, Me, i -Pr) 的分子,即甘氨酸锗酯 ( I )、α-丙氨酸 ( II ) 和L-缬氨酸 ( IV)) 两个低位异构体位于其势能面 (PES)。在β-丙氨酸酯 ( III) 中仅找到一种异构体。I和II的 IR 光谱证明在所讨论的物种中存在两种异构体,但是第二个异构体的数量从I到II显着减少。
-
Polyester carbonate copolymers, processes for preparing same and申请人:Mitsui Petrochemical Industries, Ltd.公开号:US05010146A1公开(公告)日:1991-04-23In accordance with the present invention, there are provided polyester carbonate copolymers obtained by polymerization of a mixture of 20-80% by weight of an alkylene terephthalate oligomer [I] containing ethylene terephthalate as main structural units and having an intrinsic viscosity [.eta.], as measured at 25.degree. C. in o-chlorophenol, of less than 0.6 dl/g, and 20-80% by weight of a carbonate oligomer [II] containing 2,2-bis(4 -hydroxyphenyl)propane as main structural units and having an intrinsic viscosity [.eta.], as measured at 25.degree. C. in o-chlorophenol, of less than 0.6 dl/g, said copolymers having an intrinsic viscosity [.eta.], as measured at 25.degree. C. in o-chlorophenol, of 0.4-1.2 dl/g and a glass transition temperature (Tg) of 80.degree.-140.degree. C. with a single peak, and polyester resin compositions containing said copolymers, said resin composition being excellent in transparency, heat resistance and mechanical strength.
-
새싹 식물에서 추출된 유기 게르마늄 성분이 함유된 화장품 조성물申请人:박상만公开号:KR20220014156A公开(公告)日:2022-02-04본 발명은 새싹 식물에서 추출된 유기 게르마늄 성분이 함유된 화장품 조성물에 관한 것으로서, 좀 더 상세하게는 불용성인 금속 게르마늄을 이온 상태의 유기 게르마늄으로 합성하여 식물에 대한 흡수력이 좋은 상태를 제공하기 위하여, 접하기 쉬운 산화 게르마늄의 산소 그룹을 염소 그룹으로 결합시켜 사염화게르마늄으로 합성하고, 물에 녹일 수 있는 상태인 게르마늄 이온으로 합성하기 위해 아미노산의 산(RCOOH) 그룹(또는 히드록시기(ROH))을 음이온 부분(counterpart)으로 만들어서(RCOO - , 또는 RO - ) 양이온 게르마늄(Ge 4+ )과 물에 잘 용해되는 이온상태로 합성하여, 합성된 유기 게르마늄 이온을 식물에 적용하여 유해성을 낮춘 새싹 식물에서 추출된 유기 게르마늄 성분이 함유된 화장품 조성물에 관한 것이다. 본 발명에 의하면 불용성 금속 게르마늄을 이온 상태의 유기 게르마늄으로 변화시켜 식물에 대한 흡수력이 좋은 상태로 제공함으로써 안전성과 유용성을 갖는 기능성 화장품이 제공될 수 있다.
-
Highly soluble germanium dioxide as a new source of germanium for derivatization with organic compounds作者:Artem V. Kansuzyan、Sofia D. Farafonova、Evgeniya A. Saverina、Irina V. Krylova、Victoriya A. Balycheva、Anna Ya. Akyeva、Alexander G. Medvedev、Elena N. Nikolaevskaya、Mikhail P. Egorov、Petr V. Prikhodchenko、Mikhail A. SyroeshkinDOI:10.1016/j.mencom.2022.01.007日期:2022.1In modern germanium chemistry, toxic, corrosive and water- sensitive halogen and alkoxy derivatives, or poorly reactive polymeric dioxide are generally utilized. Recently developed highly water-soluble germanium dioxide was herein treated with diols and N-donor bases to produce novel highly reactive hydrophilic germanium source suitable for further derivatization.
-
Chiral Hybrid Germanium(II) Halide with Strong Nonlinear Chiroptical Properties作者:Hebin Wang、Junzi Li、Haolin Lu、Sehrish Gull、Tianyin Shao、Yunxin Zhang、Tengfei He、Yongsheng Chen、Tingchao He、Guankui LongDOI:10.1002/anie.202309600日期:2023.10.9The first chiral germanium halide is synthesized, which exhibits strong nonlinear chiroptical property. The second-harmonic generation circular dichroism of the chiral germanium halide can reach 0.48, which is the highest value among lead-free chiral metal halides. Moreover, a large effective second-order nonlinear optical coefficient of 0.86 pm/V and a high laser-induced damage threshold of 38.46 GW/cm2
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
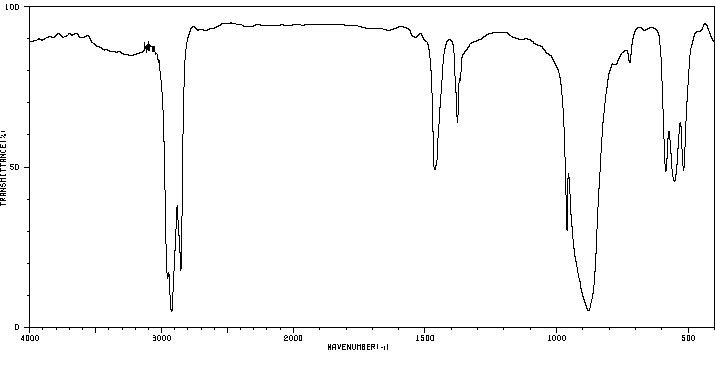
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息