氮化硼 | 10043-11-5
物质功能分类
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:2700℃
-
沸点:sublimes sl below 3000℃ [MER06]
-
密度:0.9-1.1 g/mL at 25 °C
-
溶解度:不溶于水、酸溶液
-
物理描述:DryPowder; DryPowder, OtherSolid; DryPowder, WetSolid, OtherSolid; OtherSolid
计算性质
-
辛醇/水分配系数(LogP):-0.37
-
重原子数:2
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:23.8
-
氢给体数:0
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险等级:2.1
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37
-
WGK Germany:3
-
海关编码:2850 00 20
-
危险品运输编号:UN1950
-
危险类别:2.1
-
RTECS号:ED7800000
SDS
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name BORON NITRIDE, POWDER, CA. 1 MICRON, 98%
2 - Hazards Identification
SPECIAL INDICATION OF HAZARDS TO HUMANS AND THE ENVIRONMENT
Irritating to eyes and respiratory system.
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
BORON NITRIDE 10043-11-5 233-136-6 None
Formula BN
Molecular Weight 24,8200 AMU
Synonyms BN 40SHP * Borazon * Boron mononitride * BZN 550
* Denka boron nitride GP * Denka GP * Elbor *
Elbor LO 10B1-100 * Elboron * Elbor R * Elbor RM
* Geksanit R * Hexanite R * Hexanit R * KBN-H10
* Kubonit * Kubonit KR * Sho BN * Sho BN HPS *
SP 1 * SP 1 (Nitride) * Super mighty M * UHP-Ex
* Wurzin
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If not breathing give
artificial respiration. If breathing is difficult, give oxygen.
AFTER SKIN CONTACT
In case of contact, immediately wash skin with soap and copious
amounts of water.
AFTER EYE CONTACT
In case of contact, immediately flush eyes with copious amounts
of water for at least 15 minutes.
AFTER INGESTION
ALDRICH www.molbase.com
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
5 - Fire Fighting Measures
EXTINGUISHING MEDIA
Suitable: Noncombustible. Use extinguishing media appropriate to
surrounding fire conditions.
SPECIAL RISKS
Specific Hazard(s): Emits toxic fumes under fire conditions.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear respirator, chemical safety goggles, rubber boots, and
heavy rubber gloves.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Avoid
raising dust. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
HANDLING
Directions for Safe Handling: Do not breathe dust. Avoid contact
with eyes, skin, and clothing. Avoid prolonged or repeated
exposure.
STORAGE
Conditions of Storage: Keep container closed. Store in a cool
dry place.
SPECIAL REQUIREMENTS: Hygroscopic.
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Safety shower and eye bath. Mechanical exhaust required.
GENERAL HYGIENE MEASURES
Wash thoroughly after handling.
PERSONAL PROTECTIVE EQUIPMENT
Special Protective Measures: Wear appropriate government approved
respirator, chemical-resistant gloves, safety goggles, other
protective clothing.
9 - Physical and Chemical Properties
Appearance Color: Faintly beige
Form: Powder
Property Value At Temperature or Pressure
ALDRICH www.molbase.com
pH N/A
BP/BP Range N/A
MP/MP Range N/A
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
SG/Density 2,2900 g/cm3
Partition Coefficient N/A
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
Conditions of Instability: Moisture.
Conditions to Avoid: Moisture.
Materials to Avoid: Strong oxidizing agents.
HAZARDOUS DECOMPOSITION PRODUCTS
Hazardous Decomposition Products: Nature of decomposition products
not known.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
RTECS NUMBER: ED7800000
SIGNS AND SYMPTOMS OF EXPOSURE
To the best of our knowledge, the chemical, physical, and
toxicological properties have not been thoroughly investigated.
ROUTE OF EXPOSURE
Skin Contact: May cause skin irritation.
Eye Contact: Causes eye irritation.
Inhalation: Material is irritating to mucous membranes and upper
respiratory tract.
Multiple Routes: May be harmful by inhalation, ingestion, or
skin absorption.
12 - Ecological Information
No data available.
13 - Disposal Considerations
ALDRICH www.molbase.com
SUBSTANCE DISPOSAL
Bury in a landfill site approved for the disposal of chemical and
hazardous wastes. Observe all federal, state, and local
environmental regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
Non-hazardous for air transport.
15 - Regulatory Information
CLASSIFICATION AND LABELING ACCORDING TO EU DIRECTIVES
INDICATION OF DANGER: Xi
Irritant.
R-PHRASES: 36/37
Irritating to eyes and respiratory system.
S-PHRASES: 26-36
In case of contact with eyes, rinse immediately with plenty of
water and seek medical advice. Wear suitable protective
clothing.
COUNTRY SPECIFIC INFORMATION
Germany
WGK: 3
Self-Classification
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
氮化硼是由氮原子和硼原子构成的晶体,主要分为三种:六方氮化硼(HBN)、密排六方氮化硼(WBN)以及立方氮化硼。其中,六方氮化硼拥有类似石墨层状结构,呈现白色粉末状,质地松散、润滑,易吸潮且质轻,因此被称作“白色石墨”。
六方氮化硼的膨胀系数与石英相近,但导热率是石英的十倍。它在高温时表现出良好的润滑性,是一种优质的高温固体润滑剂。此外,六方氮化硼还具有很强的中子吸收能力,并且化学性质稳定,对几乎所有熔融金属都是惰性的。
六方氮化硼不溶于冷水,但在热水中水解缓慢并产生少量硼酸和氨。它与弱酸和强碱在室温下不起反应,在热酸中微溶。在熔融的氢氧化钠或氢氧化钾作用下可以分解。此外,六方氮化硼对各种无机酸、碱、盐溶液及有机溶剂具有相当的抗腐蚀能力。
立方氮化硼立方氮化硼是一种超硬材料,硬度仅次于金刚石,并于1957年由美国的R.H.温托夫首次研制成功。它不仅拥有与金刚石相似的良好特性,还具备更高的热稳定性和对铁族金属的化学稳定性。广泛应用于加工淬火钢、冷硬铸铁和耐热合金等黑色金属。
化学性质 用途氮化硼因其多种优良性能而被广泛应用:
- 制造耐火材料和炉子绝缘材料
- 应用于电子、机械及航空工业等领域
- 作为高压高频电及等离子弧的绝缘体,自动焊接耐高温架涂层,高频感应电炉的材料,半导体固相掺和料,原子反应堆结构材料,防止中子辐射的包装材料,雷达传递窗,雷达天线介质以及火箭发动机组成物。
- 由于其优良的润滑性能,可作为高温润滑剂和多种模具脱模剂
- 模压氮化硼可用于制造耐高温坩埚和其他制品,适用于地质勘探、石油钻探钻头及高速切削工具等金属加工研磨材料。此外,还能用作各种材料的添加剂。
- 由氮化硼加工制成的纤维为中模数高功能纤维,是一种无机合成工程材料,在化学工业、纺织工业、宇航技术及其他尖端行业均有广泛用途。
氮化硼还可用作:
生产方法立方晶型氮化硼的制造方法包括:
上下游信息
反应信息
-
作为反应物:参考文献:名称:Gmelin Handbuch der Anorganischen Chemie, Gmelin Handbook: B: MVol., 7.2, page 109 - 111摘要:DOI:
-
作为产物:参考文献:名称:在离子液体中脱氢制备氮化硼和聚苯乙烯/氮化硼复合颗粒摘要:氮化硼(BN)是通过在低于200℃的离子液体(1-丁基-3-甲基咪唑四氟硼酸酯,[Bmim] [BF 4 ])中于300℃以下温度将硼硼烷(AB)脱氢制得的。 BN的一般制备方法,低于聚苯乙烯(PS)的分解温度。反应在大气压下于120℃下进行10小时,随后将产物材料在[Bmim] [BF 4 ]中在减压下于300℃下加热24小时。与在本体系统(无溶剂系统)中观察到的相比,使用[Bmim] [BF 4 ]作为介质时,反应速率和最终转化率增加。此外,在[Bmim] [BF]中通过脱氢成功制备了PS / BN复合颗粒4 ]在交联的PS种子颗粒的存在下。复合颗粒超薄截面的透射电镜图像证实了具有PS核和BN壳的颗粒的核-壳形态。DOI:10.1039/c3ra47722c
-
作为试剂:描述:参考文献:名称:一种利用半导体光催化加氢还原有机卤化物 的方法摘要:本发明公开了一种利用半导体光催化加氢还原有机卤化物的方法,其是以半导体材料硼氮碳为光催化剂,在室温、可见光光照条件下,将有机卤化物C‑X(X=Br,Cl)加氢还原为脱卤化合物。本发明将硼氮碳用于加氢还原有机卤化物,其反应过程操作简单,催化效果好,且在可见光下即可进行,条件温和,成本低,符合实际生产需要,具有较大的应用潜力。公开号:CN108440236B
文献信息
-
Phase transitions in Ca3(BN2)2 and Sr3(BN2)2作者:Marco Häberlen、Jochen Glaser、H.-Jürgen MeyerDOI:10.1016/j.jssc.2005.02.008日期:2005.5α-Ca3(BN2)2 crystallizes in the cubic system (space group: Im3¯m) with one type of calcium ions disordered over 78 of equivalent (8c) positions. An ordered low-temperature phase (β-Ca3(BN2)2) was prepared and found to crystallize in the orthorhombic system (space group: Cmca ) with lattice parameters: a=1024.18(2)pm, b=732.43(2)pm, and c=2091.60(4)pm. Structure refinements on the basis of X-ray powderα- Ca 3(BN 2)2在立方体系(空间群:Im3m )中结晶,其中一种钙离子在78个等价(8 c)位置上无序排列。制备了有序的低温相(β - Ca 3(BN 2)2),发现该晶体在正交晶系(空间群:Cmca )中以晶格参数a = 1024.18(2)pm ,b = 732.43(2)结晶。)pm和c = 2091.60(4)pm 。在X射线粉末数据的基础上对结构进行了细化,发现正交晶β- Ca 3(BN 2)图2对应于立方α - Ca 3(BN 2)2的有序超结构。分配给β- Ca 3(BN 2)2的空间群Cmca是通过群-子群关系从Im3¯m推导出来的。
-
Unsymmetrische BN<sub>2</sub> <sup>3-</sup>-Ionen in den Strukturen von Ca<sub>2</sub>ClBN<sub>2</sub> und Sr<sub>2</sub>ClBN<sub>2</sub> / Unsymmetric BN<sub>2</sub> <sup>3-</sup>-Ions in the Structures of Ca<sub>2</sub>ClBN<sub>2</sub> and Sr<sub>2</sub>ClBN<sub>2</sub>作者:Olaf Reckeweg、H.-Jürgen MeyerDOI:10.1515/znb-1997-0307日期:1997.3.1
Abstract The new compounds Ca2ClBN2 (1) and Sr2ClBN2 (2) were prepared from the respective metal, its dihalide and h-BN in sealed tantalum ampoules at 1200 °C. The crystals obtained were transparent yellow (1) and blue (2), respectively. The crystal structures were determined from single crystal X-ray data. Ca2ClBN2 and Sr2ClBN2 are isotypic and crystallize in the orthorhombic space group Pnma (No. 62), Z = 4 (Ca2ClBN 2: a = 1166.7(2), b = 390.26(4), c = 899.8(1) pm, R1 = 0.043, wR2 = 0.115 for 554 independent reflections; Sr2ClBN2: a = 1242.8(1), b = 416.75(4), c = 920.8(1) pm, R1 = 0.031, wR2 = 0.054 for 662 independent reflections).
The structures contain two different layers of M2+, Cl- and BN2 3- alternating along the [010] direction. The bond angles N-B-N are 177.2(4)° for (1) and 176,6(5)° for (2), the bond distances of the BN2 3- ions are dB_ N1] = 134.6(5) pm for (1), 136,3(7) pm for (2) and dB_N2 = 132.4(5) pm for (1) and 131,3(7) pm for (2). The unsymmetric structure of the BN2 3- ion, as is manifested particularly in the Sr compound (2), is caused by the coordination of N1 to four cations while N2 is coordinated only to three.
新化合物Ca2ClBN2(1)和Sr2ClBN2(2)分别由金属、其二卤化物和h-BN在密封的钽安瓿中在1200°C下制备而成。所得到的晶体分别为透明的黄色(1)和蓝色(2)。晶体结构是根据单晶X射线数据确定的。Ca2ClBN2和Sr2ClBN2是同型的,并且结晶在正交晶系空间群Pnma(No. 62)中,Z = 4(Ca2ClBN2:a = 1166.7(2)、b = 390.26(4)、c = 899.8(1)皮米,R1 = 0.043,wR2 = 0.115,对于554个独立反射;Sr2ClBN2:a = 1242.8(1)、b = 416.75(4)、c = 920.8(1)皮米,R1 = 0.031,wR2 = 0.054,对于662个独立反射)。 这些结构包含沿[010]方向交替的两个不同层的M2+、Cl-和BN23-。N-B-N键角分别为177.2(4)°(1)和176.6(5)°(2),BN23-离子的键距为d[B_N1] = 134.6(5)皮米(1)、136.3(7)皮米(2)和d[B_N2] = 132.4(5)皮米(1)、131.3(7)皮米(2)。BN23-离子的非对称结构,特别是在Sr化合物(2)中表现出来,是由于N1与四个阳离子配位,而N2只与三个阳离子配位。 -
Rare-Earth Nickel Nitridoborates with (BN) Anions: CharacterizedRENi(BN) and AnticipatedREM(BN) Compounds作者:Michael Neukirch、Björn Blaschkowski、Marco Häberlen、H.-Jürgen MeyerDOI:10.1002/zaac.200600004日期:2006.8The compounds RENi(BN) with RE = Y, La, Ce, Pr, Tm and Yb were synthesized by solid state reactions from metal powders and α-BN at temperatures between 1000° and 1200 °C. In addition, the mixed phases (Ca,Yb)Ni(BN) and (Ca,Tm)Ni(BN) were synthesized. All products were characterized by powder XRD measurements and indexed isotypic with the structure of CaNi(BN), consistent with the tetragonal space group
-
High-Yield Synthesis of B4C/BN Ceramic Materials by Pyrolysis of Polymeric Lewis Base Adducts of Decaborane(14)作者:William Smith Rees、Dietmar SeyferthDOI:10.1111/j.1151-2916.1988.tb05873.x日期:1988.4Polymers of type [B10H12˙diamine]x (diamine=H2NCH2CH2NH2, (CH3)2NCH2CH2NH2, (CH3)2NCH2CH2N(CH3)2, etc.) have been found to be useful ceramic precursors. In a stream of argon, their pyrolysis gives B4C/BN; in a stream of ammonia, BN.
-
Synthesis of Boron Phosphide and Nitride作者:R. C. VICKERYDOI:10.1038/184268a0日期:1959.7A NOVEL technique has been found for the synthesis of boron nitride and phosphide involving thermal decomposition of halide addition compounds. Further work is intended on the reactions involved but the information thus far obtained is felt to warrant a brief, preliminary report for the benefit of other workers in this field.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
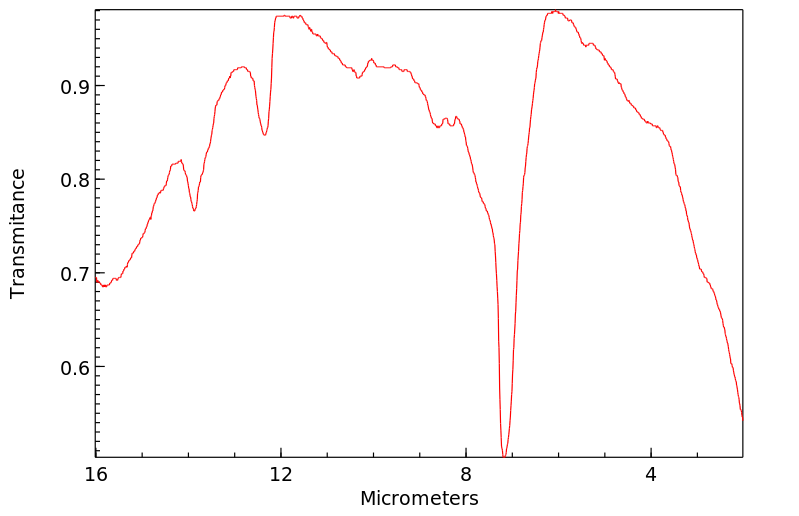
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息