四乙基铅 | 78-00-2
分子结构分类
中文名称
四乙基铅
中文别名
4-乙酰氧基苯甲醛;四乙鉛;四乙铅
英文名称
tetraethyllead(IV)
英文别名
Tetraaethylblei;Tetraethylblei;Bleitetraethyl;Bleitetraaethyl;tetraethyllead;Tetraaethyl-plumban;Tetraethyl-plumban
CAS
78-00-2
化学式
C8H20Pb
mdl
——
分子量
323.447
InChiKey
MRMOZBOQVYRSEM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
稳定性/保质期:
- 稳定性[23]:稳定。
- 禁配物[24]:强氧化剂、强酸、强碱。
- 应避免的条件[25]:受热。
- 聚合危害[26]:不聚合。
- 分解产物[27]:铅化物。
计算性质
-
辛醇/水分配系数(LogP):3.51
-
重原子数:9
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
代谢
After iv injection into rats, tetraethyl lead (TEL) is converted into triethyl lead, which is considered to be responsible for toxic effects seen. ... After iv injections of 25 mg/kg bw of TEL into rabbits, main metabolite was triethyl lead ...
来源:Hazardous Substances Data Bank (HSDB)
代谢
Dealkylation of tetraethyl lead occurs in microsomes and requires oxygen and NADPH, and has been observed in homogenates of liver, kidney, and brain of rat and rabbit.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Biological degradation of tetraethyllead to the triethyllead cation by rat liver microsomes from untreated, phenobarbital pretreated and methylcholanthrene pretreated rats was studied; nicotinamide-adenine dinucleotide phosphate and oxygen are essential.
来源:Hazardous Substances Data Bank (HSDB)
代谢
烟酸腺嘌呤二核苷酸磷酸和氧气依赖的微粒体代谢铅的四乙基取代衍生物在大鼠中产生了乙烯作为主要产物和乙烷作为次要产物。这些反应是由肝微粒体细胞色素P450依赖的单加氧酶催化的。由于无氧条件对乙烷和乙烯的形成有不同的抑制作用,结果表明大部分产生的乙烷是通过还原机制衍生而来的。
Nicotinamide-adenine dinucleotide phosphate and oxygen dependent microsomal metabolism of tetraethyl-substituted derivatives of lead gave rise to ethylene as a major product and ethane as minor product in rats. Reactions were catalyzed by liver microsomal cytochrome P450 dependent monooxygenase. Since formation of ethane and ethylene was differentially inhibited by anaerobiosis, results suggested that a large portion of the ethane produced was derived by a reductive mechanism.
来源:Hazardous Substances Data Bank (HSDB)
代谢
铅通过吸入、口服和皮肤接触被吸收,然后主要分布到骨骼和红细胞中。在血液中,铅可能被发现与血清白蛋白或金属结合蛋白金属lothionein结合。有机铅通过细胞色素P-450酶代谢,而无机铅则与δ-氨基酮酸脱水酶形成复合物。铅主要通过尿液和粪便排出。
Lead is absorbed following inhalation, oral, and dermal exposure. It is then distributed mainly to the bones and red blood cells. In the blood lead may be found bound to serum albumin or the metal-binding protein metallothionein. Organic lead is metabolized by cytochrome P-450 enzymes, whereas inorganic lead forms complexes with delta-aminolevulinic acid dehydratase. Lead is excreted mainly in the urine and faeces. (L136)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
铅模仿其他生物学上重要的金属,如锌、钙和铁,作为许多它们各自酶促反应的辅因子与之竞争。例如,铅已被证明能竞争性地抑制钙与钙调蛋白的结合,干扰神经递质的释放。它对NMDA受体和蛋白激酶C表现出类似的竞争性抑制,这损害了脑微血管的形成和功能,并改变了血脑屏障。铅还通过阻碍多巴胺合成的调节和阻止乙酰胆碱的诱发释放来影响神经系统。然而,其主要作用机制是通过抑制δ-氨基酮酸脱水酶,这是一种在血红素生物合成中至关重要的酶,而血红素是血红蛋白所需的必要辅因子。(T4, A20, A22, L136)
Lead mimics other biologically important metals, such as zinc, calcium, and iron, competing as cofactors for many of their respective enzymatic reactions. For example, lead has been shown to competitively inhibit calcium's binding of calmodulin, interferring with neurotransmitter release. It exhibits similar competitive inhibition at the NMDA receptor and protein kinase C, which impairs brain microvascular formation and function, as well as alters the blood-brain barrier. Lead also affects the nervous system by impairing regulation of dopamine synthesis and blocking evoked release of acetylcholine. However, it's main mechanism of action occurs by inhibiting delta-aminolevulinic acid dehydratase, an enzyme vital in the biosynthesis of heme, which is a necesssary cofactor of hemoglobin. (T4, A20, A22, L136)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
对于无机铅化合物的致癌性,人类中的证据有限。对于有机铅化合物的致癌性,人类中的证据不足。在实验动物中,有足够的证据表明无机铅化合物具有致癌性……在实验动物中,对于有机铅化合物的致癌性证据不足。在实验动物中,对于四乙铅的致癌性证据不足……无机铅化合物可能对人类具有致癌性(2A组)。有机铅化合物对人类的致癌性无法分类(3组)。工作组指出,有机铅化合物至少部分在人类和动物体内代谢为离子铅。由于有机铅产生的离子铅存在于体内,预计它将表现出与无机铅相关的毒性。
There is limited evidence in humans for the carcinogenicity of inorganic lead cmpd. There is inadequate evidence in humans for the carcinogenicity of organic lead cmpd. There is sufficient evidence in exptl animals for the carcinogenicity of inorganic lead cmpd ... There is inadequate evidence in exptl animals for the carcinogenicity of organic lead cmpd. There is inadequate evidence in exptl animals for the carcinogenicity of tetraethyl lead ... Inorganic lead cmpd are probably carcinogenic to humans (Group 2A). Organic lead cmpd are not classifiable as to their carcinogenicity to humans (Group 3). The working group noted that organic lead cmpd are metabolized, at least in part, to ionic lead both in humans and animals. To the extent that ionic lead, generated from organic lead, is present in the body, it will be expected to exert the toxicities associated with inorganic lead.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
A4:不能分类为人类致癌物。/如铅/
A4: Not classifiable as a human carcinogen. /As Pb/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
Lead, lead compounds: Reasonably anticipated to be a human carcinogen
来源:Hazardous Substances Data Bank (HSDB)
毒理性
Organic lead compounds are not classifiable as to their carcinogenicity to humans (Group 3). To the extent that organic lead compounds are metabolized in part to ionic lead, they are expected to exert the toxicities associated with inorganic lead (Group 2A, probably carcinogenic to humans). (L135)
来源:Toxin and Toxin Target Database (T3DB)
吸收、分配和排泄
有机铅化合物,如四乙铅和四甲基铅,在呼吸道中表现为气体,并且比无机铅颗粒的吸收程度要高。有机铅化合物也可以通过人类和实验动物的皮肤吸收。四乙铅和四甲基铅在体内发生氧化脱烷基反应。任何内源产生的无机铅的分布模式与摄入的无机铅相同,但是母体化合物和中间脱烷基产物分布得相当不同,并且与它们的亲脂性相符。在暴露于四乙铅的人类中,母体化合物及其代谢物(包括无机铅)在肝脏和肾脏中的浓度最高,其次是大脑和心脏……在暴露于烷基铅的大鼠中,总铅浓度最高的器官是肾脏和肝脏,其次是大脑。在人类中,四乙铅被发现以二乙铅和无机铅的形式通过尿液排出。在大鼠和兔子中,尿液中的主要代谢物是二烷基铅。四烷基铅也会以无机铅的形式通过粪便排出,这是代谢的最终产物。在人类中,通过肺部呼出四乙铅和四甲基铅是主要的排泄途径,在吸入后48小时,分别占吸入剂量的40%(四甲基铅)和20%(四乙铅)。
Organic lead cmpd, such as tetraethyl lead and tetramethyl lead, behave as gases in the respiratory tract, and are absorbed to a greater extent than are inorganic lead particles. Organic lead cmpd are also absorbed through the skin of both humans and exptl animals. Tetraethyl lead and tetramethyl lead are oxidatively dealkylated in the body. Any inorganic lead produced endogenously is distributed in the same pattern as admin inorganic lead, but the parent cmpd and the intermediate dealkylated products are distributed quite differently and in accordance with their lipophilicity. In humans exposed to tetraethyl lead, concn of the parent cmpd and its metabolites, including inorganic lead, are highest in the liver and kidneys followed by the brain and heart ... The highest concn of total lead in rats after exposure to alkyl leads are found in the kidney and liver, followed by the brain. In humans, tetraethyl lead was found to be excreted in the urine as diethyl lead and inorganic lead. In rats and rabbits, dialkyl lead is the major metabolite found in urine. Tetraalkyl leads would also be excreted in the feces as inorganic lead, the end product of metabolism. In humans, exhalation of tetraethyl lead and tetramethyl lead from the lung is a major route of excretion, accounting for 40% (tetramethyl lead) and 20% (tetraethyl lead) of the inhaled dose at 48 hr after inhalation..
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
Seven victims of accidental tetraalkyllead poisoning died 5-19 days after poisoning. The highest lead concentration was detected in spleen, liver and kidney tissue.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
在接触了致死剂量的四乙铅(TEL)或四甲基铅(TML)的大鼠和狗,以及因TEL中毒致命的人的肺部、大脑、肝脏和肾脏组织中,铅(Pb)的含量为0.7-13.0毫克/100克组织,这一数据适用于三种物种。人脑、肝脏和肾脏中的铅含量与大鼠和狗相应组织中的含量相似。
Tissue distribution studies of lead in rats and dogs exposed to lethal inhalation doses of tetraethyl lead (TEL) or tetramethyl lead (TML) and in men fatally poisoned by TEL revealed lead (Pb) levels of 0.7-13.0 mg/100 g tissue in lung, brain, liver and kidney in three species. Human Pb levels in brain, liver and kidney resembled those seen in corresponding rat and dog tissues.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
In cases of accidental poisoning with tetraethyl lead (TEL), liver, kidney, pancreas, brain and heart accumulate triethyllead, and total tissue lead (Pb) concentration correlate with triethyllead concentrations in corresponding tissues.
来源:Hazardous Substances Data Bank (HSDB)
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 二乙基二甲基铅 Diethyl-dimethyl-plumbane 1762-27-2 C6H16Pb 295.393 乙基三甲基铅 ethyltrimethylplumbane 1762-26-1 C5H14Pb 281.366 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 三乙基甲基铅 triethyl-methyl plumbane 1762-28-3 C7H18Pb 309.42 二乙基二甲基铅 Diethyl-dimethyl-plumbane 1762-27-2 C6H16Pb 295.393 乙基三甲基铅 ethyltrimethylplumbane 1762-26-1 C5H14Pb 281.366
反应信息
-
作为反应物:参考文献:名称:Goddard,A.E.; Goddard,D., Journal of the Chemical Society, 1922, vol. 121, p. 260摘要:DOI:
-
作为产物:参考文献:名称:Preparation of organic lead compounds摘要:公开号:US02850513A1
-
作为试剂:参考文献:名称:Vaughan; Rust, Journal of Organic Chemistry, 1942, vol. 7, p. 474摘要:DOI:
文献信息
-
Preparation of tetra alkyl lead申请人:DU PONT公开号:US01798593A1公开(公告)日:1931-03-31
279,106. Daudt, H. W. Oct. 15, 1926, [Convention date]. Lead tetraethyl is prepared by the action of magnesium ethyl chloride upon lead salts. In an example, ethyl chloride is added to magnesium turnings in the presence of ether containing a small proportion of methyl iodide and a crystal of iodine. The resulting solution of magnesium ethyl chloride is then treated with a suspension of lead chloride in ether for some hours. The product is poured into water, the ether distilled off, and the lead tetraethyl obtained by steam distillation. The Specification as open to inspection under Sect. 91 (3) (a) comprises also the use of alkyl iodides other than methyl iodide. This subjectmatter does not appear in the Specification as accepted.
-
Oxidative carbonylation of hydroxyaromatic compounds申请人:General Electric Company公开号:US06462217B1公开(公告)日:2002-10-08Organolead compounds such as tetraethyllead are useful in catalyst compositions for the oxidative carbonylation of hydroxyaromatic compounds to diaryl carbonates. They are employed in combination with a Group 8, 9, or 10 metal such as palladium, or a compound thereof, and a bromide or chloride such as tetraethylammonium bromide.
-
Ready coupling of acid chlorides with tetra-alkyl-lead derivatives catalysed by palladium
-
Norbornyl Cations of Group 14 Elements作者:Thomas Müller、Christian Bauch、Markus Ostermeier、Michael Bolte、Norbert AunerDOI:10.1021/ja021234g日期:2003.2.1(4i)) and the small J coupling constants between the element and the remote vinyl carbons in the case of 4h and 4i (J(CSn) = 26 Hz, J(CPb) = 16 Hz) give experimental evidence for the intramolecular interaction and the charge transfer between the positively charged element and the remote C=C double bond. The experimental results are supported by quantum mechanical calculations of structures, energies, and14 族元素 Si --> Pb 的降冰片基阳离子已从取代的 3-环戊烯甲基前体通过将瞬时阳离子分子内加成到 3-环戊烯甲基取代基的 C=C 双键(pi-路线到降冰片基阳离子)合成。降冰片基阳离子 4a (E = Si, R = Me), 4e (E = Si, R = Et), 4f (E = Si, R = Bu), 4g (E = Ge, R = Bu), 4h (E = Sn, R = Bu) 和 4i (E = Pb, R = Et) 已通过其特征 NMR 化学位移 (4a,e,f, delta((29)Si) = 80-87, delta((( 13)C)(CH=) = 149.6-150.6; 4g, delta((13)C)(CH=) = 144.8; 4h, delta((119)Sn) = 334, delta((13)C)(CH =) = 141.5; 4i, delta((207)Pb)
-
Synthesis of a non-symmetric azodicarbonyl compound and its regioselective reaction with organometallic reagents作者:Yoshinori Yamamoto、Masatoshi Yumoto、Jun-ichi YamadaDOI:10.1016/0040-4039(91)80694-2日期:1991.6The non-symmetric azodicarbonyl compound 7 reacted with R-M/Lewis acid regioselectively at the nitrogen atom attached to the amide group giving 9, whereas it reacted with R-M at the nitrogen atom attached to the ester group producing 8 in high yield.非对称的偶氮二羰基化合物7在与酰胺基连接的氮原子上与RM /路易斯酸区域选择性反应,得到9,而与在酯基连接的氮原子上的RM发生区域选择性反应,从而高产率地生成8。
表征谱图
-
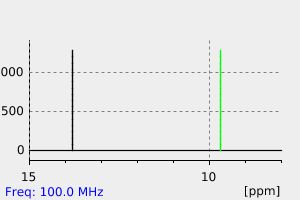
氢谱1HNMR
-
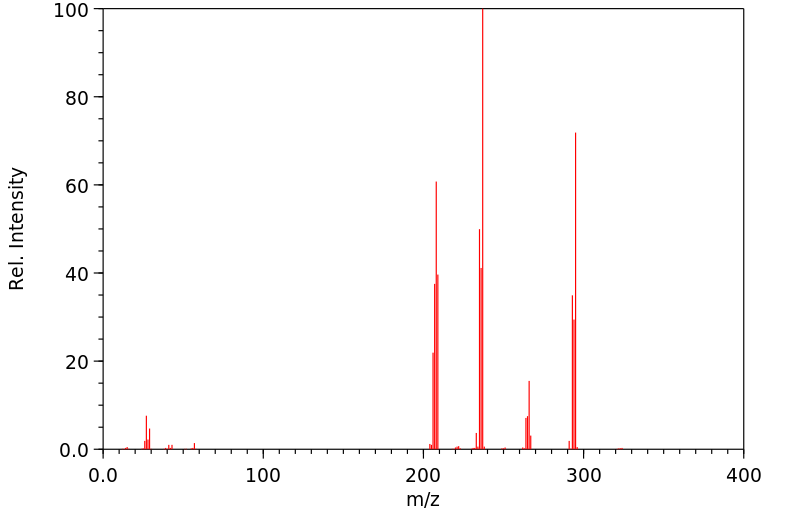
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
锰,五羰基(三甲基甲锡烷基)-,(OC-6-22)-
锡烷,乙氧基三甲基-
锡烷,三丁基(1E)-1-庚烯基-
锡烷,三丁基(1-甲基-2-丁烯基)-,(Z)-
锡烷,三(1,1-二甲基乙基)乙炔基-
锡烷,(4-氯二环[2.2.1]庚-1-基)三甲基-
锡烷,(1E)-1-丁烯-3-炔基三丁基-
铝,三庚基-
铝,丁氧基二(2-甲基丙基)-
铅烷,三丁基-1-己炔基-
辛基锡
辛基氧代锡烷
膦,三(三甲基甲锡烷基)-
碳化铝
碘化三乙基铅
碘(三甲基)铅烷
硼烷胺,N,N-二(氯二甲基甲锡烷基)-1,1-二甲基-
硫烷负离子三甲基铅
硫代乙酸 S-[3-(三丁基锡烷基)丙基]酯
硒基二(三甲基锡)
癸酰(二羟基)铝
甲硫基三丁基锡烷
甲烷四基四(三甲基锡烷)
甲氧基二(2-甲基丙基)-铝
甲基锡
甲基烯丙基三正丁基锡
甲基氢化钼
甲基双(1-甲基环己基)锡烷
甲基二氯化铝
甲基三戊基锡
甲基(三丙基)锡烷
环己羧酸,2-氨基-,甲基酯,(1S,2S)-
环己基三异丙基锡烷
环己基[(三丁基锡烷基)氧基]重氮1-氧化物
环己基-三甲基锡烷
环己基(异丙基)二甲基锡烷
环丙基(三异丙基)锡烷
烯丙基三甲基锡烷
烯丙基三乙烯基锡烷
烯丙基三丁基锡
烯丙基三(3,3,4,4,5,5,6,6,7,7,8,8,8-十三氟辛基)锡烷
溴二乙基铝
溴三甲基铅
溴(异丙基)汞
溴(三乙基)铅
溴(三丁基)铅
氰酸三丁基锡烷
氯甲氧基甲基三丁基锡
氯甲基三甲基锡
氯化二己基铝