2,2,6,6-四甲基-4-哌啶酮肟 | 4168-79-0
中文名称
2,2,6,6-四甲基-4-哌啶酮肟
中文别名
N-羟基-2,2,6,6-四甲基哌啶-4-亚胺;2,2,6,6-四甲基-4-二乙哌啶二酮肟;2,2,6,6-四氟-1,4-二氮杂二环[2.2.2]辛烷
英文名称
4-hydroxyimino-2,2,6,6-tetramethylpiperidine
英文别名
2,2,6,6-tetramethyl-4-oxiiminopiperidine;2,2,6,6-tetramethylpiperidin-4-one oxime;2,2,6,6-tetramethyl-4-oximinopiperidine;2,2,6,6-tetramethylpiperidin-4-oxime;triacetone amine oxime;4-hydroxyimino-TMPD;2,2,6,6-Tetramethyl-4-piperidone oxime;N-(2,2,6,6-tetramethylpiperidin-4-ylidene)hydroxylamine
CAS
4168-79-0
化学式
C9H18N2O
mdl
MFCD00234585
分子量
170.255
InChiKey
FFUVOHCLAIYVTL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:153°C
-
沸点:299.91°C (rough estimate)
-
密度:1.0114 (rough estimate)
-
稳定性/保质期:
在常温常压下稳定,熔点为153°C。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:12
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.888
-
拓扑面积:44.6
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险类别码:R36/37/38
-
储存条件:常温下应存于避光、通风且干燥的地方,并密封保存。
SDS
2,2,6,6-四甲基-4-哌啶酮肟 修改号码:5
模块 1. 化学品
产品名称: 2,2,6,6-Tetramethyl-4-piperidone Oxime
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第4级
急性毒性(经皮) 第4级
急性毒性(吸入) 第4级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 吸入或皮肤接触或吞咽有害。
防范说明
[预防] 避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。若感不适,呼叫解毒
中心/医生。
食入:若感不适,呼叫解毒中心/医生。漱口。
皮肤接触:用大量肥皂和水轻轻洗。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
2,2,6,6-四甲基-4-哌啶酮肟 修改号码:5
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2,2,6,6-四甲基-4-哌啶酮肟
百分比: ....
CAS编码: 4168-79-0
俗名: Triacetonamine Oxime
分子式: C9H18N2O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 一旦发生火警,有爆炸的危险。由于爆炸的危险,远距离灭火。
小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。切勿引起泄漏、溢出或分散。切勿产
生不必要的蒸气。远离热源/火花/明火/热表面。禁烟。采取措施防止静电积累。使用
防爆设备。避免冲击和摩擦。处理后彻底清洗双手和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
确保容器不会受到未测影响,比如跌落或脱落。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
2,2,6,6-四甲基-4-哌啶酮肟 修改号码:5
模块 8. 接触控制和个体防护
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 153°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 热、撞击、摩擦等条件下可能爆炸分解。
避免接触的条件: 热, 冲击, 摩擦
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: ivn-mus LD50:180 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: TO0128000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
2,2,6,6-四甲基-4-哌啶酮肟 修改号码:5
模块 12. 生态学信息
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门和专家。建议在可燃溶剂中溶解混合,然后在装有后燃和洗涤装置的化
学焚烧炉中慢慢焚烧。如果一次性焚烧大量物质,可能发生爆炸。废弃处置时请遵守国家、地区和当地的所有法规
。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2,2,6,6-Tetramethyl-4-piperidone Oxime
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第4级
急性毒性(经皮) 第4级
急性毒性(吸入) 第4级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 吸入或皮肤接触或吞咽有害。
防范说明
[预防] 避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。若感不适,呼叫解毒
中心/医生。
食入:若感不适,呼叫解毒中心/医生。漱口。
皮肤接触:用大量肥皂和水轻轻洗。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
2,2,6,6-四甲基-4-哌啶酮肟 修改号码:5
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2,2,6,6-四甲基-4-哌啶酮肟
百分比: ....
CAS编码: 4168-79-0
俗名: Triacetonamine Oxime
分子式: C9H18N2O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 一旦发生火警,有爆炸的危险。由于爆炸的危险,远距离灭火。
小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。切勿引起泄漏、溢出或分散。切勿产
生不必要的蒸气。远离热源/火花/明火/热表面。禁烟。采取措施防止静电积累。使用
防爆设备。避免冲击和摩擦。处理后彻底清洗双手和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
确保容器不会受到未测影响,比如跌落或脱落。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
2,2,6,6-四甲基-4-哌啶酮肟 修改号码:5
模块 8. 接触控制和个体防护
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 153°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 热、撞击、摩擦等条件下可能爆炸分解。
避免接触的条件: 热, 冲击, 摩擦
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: ivn-mus LD50:180 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: TO0128000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
2,2,6,6-四甲基-4-哌啶酮肟 修改号码:5
模块 12. 生态学信息
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门和专家。建议在可燃溶剂中溶解混合,然后在装有后燃和洗涤装置的化
学焚烧炉中慢慢焚烧。如果一次性焚烧大量物质,可能发生爆炸。废弃处置时请遵守国家、地区和当地的所有法规
。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:2,2,6,6-四甲基-4-哌啶酮肟 在 1-癸磺过氧酸 作用下, 以 氯仿 为溶剂, 以40%的产率得到4-hydroxyimino-2,2,6,6-tetramethylpiperidin-1-yloxyl参考文献:名称:The oxidation of secondary amines by alkanesulfonic peracids摘要:Alkanesulfonic peracids are efficient oxidation agents for the conversion of secondary amines to the corresponding nitroxyl radicals.DOI:10.1007/bf00961711
-
作为产物:描述:参考文献:名称:六种荧光素-硝基氧自由基杂化化合物的合成及荧光性质。摘要:通过5-或6-羧基荧光素与4-氨基-TEMPO(2ab),5-或6-氨基荧光素和4的缩合反应,合成了六种荧光素-硝基氧自由基杂化化合物(2ab,3ab,4和5)。 -羧基-TEMPO(3ab),以及荧光素和4-羧基-TEMPO(4),或使荧光素的3-羟基与DPROXYL-3-基甲基甲磺酸酯反应(5)。自由基还原后的荧光强度(约520nm)分别为2a,2b和3b的1.43倍,1.38倍和1.61倍;单独的3a显示出还原强度降低。由于4容易溶于PBS或什至甲醇中以提供荧光素和4-羧基-TEMPO,因此无法测量其荧光变化。在荧光素酚和3-羟甲基-DPROXYL羟基中心之间包含醚键的杂化化合物5是稳定的,并且在还原时,DOI:10.1016/j.saa.2016.06.021
文献信息
-
Reactions of nitroxides. Part X: Antifungal activity of selected sulfur and selenium derivatives of 2,2,6,6-tetramethylpiperidine作者:Jerzy Zakrzewski、Maria KrawczykDOI:10.1016/j.bmcl.2010.10.092日期:2011.1The antifungal activity of nitroxyl radicals—derivatives of 2,2,6,6-tetramethylpiperidine-1-oxyl with reactive substituents 4-isothiocyanato-, 4-isocyano-, and 4-isoselenocyanato- and of -formyl-, -thioformyl-, -selenoformyl-derivatives of 2,2,6,6-tetramethylpiperidine was investigated. Those of the above compounds, which contain a sulfur or selenium atom are the most active against four fungus plant
-
Selective synthesis of dimethyl ketone oxime through ammoximation over Ti-MOR catalyst作者:Jianghong Ding、Peng WuDOI:10.1016/j.apcata.2014.09.038日期:2014.11was highly active and selective for the liquid-phase ammoximation of dimethyl ketone (DMK) with ammonia and hydrogen peroxide. The parameters effecting the formation of the ammoximation product of dimethyl ketone oxime were investigated systematically in a batch-type reactor, and the optimized conditions were further applied to continuous ammoximation of DMK in a slurry reactor. Ti-MOR was superior to
-
Electrochemical halogenation of 4-hydroximino-2,2,6,6-tetramethylpiperidine
-
Acetylene based short route from 2,2,6,6-tetramethylpiperidin-4-one oxime to 2-(pyrazol-5-yl)-4,5,6,7-tetrahydropyrrolo[3,2-c]pyridines作者:Elena F. Sagitova、Denis N. Tomilin、Olga V. Petrova、Arsalan B. Budaev、Lyubov N. Sobenina、Boris A. Trofimov、Guoqiang Q. Yang、Rui HuDOI:10.1016/j.mencom.2019.11.018日期:2019.11Pharmaceutically relevant substituted 2-(pyrazol-5-yl)-4,5,6,7-tetrahydropyrrolo [3,2-c]pyridines have been assembled in good to excellent yields via the reaction of 2,2,6,6-tetramethylpiperidin-4-one oxime with acetylene, cross-coupling of the resulting 4,4,6,6-tetramethyl-4,5,6,7 tetrahydropyrrolo[3,2-c]pyridines with aroylbromoacetylenes, and reaction of the formed 2-(aroylethynyl)-4,4,6,6-tetramethyl-4
-
Polymer stabilizers containing both hindered amine and hydroxlamine申请人:Ciba-Geigy Corporation公开号:US05410047A1公开(公告)日:1995-04-25Compounds possessing both a hindered amine moiety, such as a derivative of 2,2,6,6-tetramethylpiperidine, and a hydroxylamine moiety where the N-hydroxy group is not attached to a nitrogen atom in a piperidine ring are valuable stabilizers for protecting polymer compositions against the deleterious effects of actinic light and from the adverse effects of high temperature polymer processing environments.同时具有受阻胺基团(如2,2,6,6-四甲基哌啶衍生物)和羟肟基团的化合物,其中N-羟基基团未附着在哌啶环中的氮原子上,对于保护聚合物组合物免受光化学反应和高温聚合物加工环境的不利影响是有价值的稳定剂。
表征谱图
-
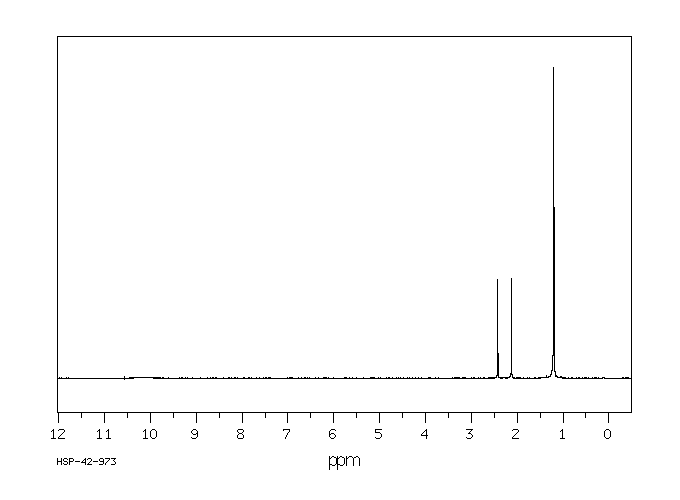
氢谱1HNMR
-
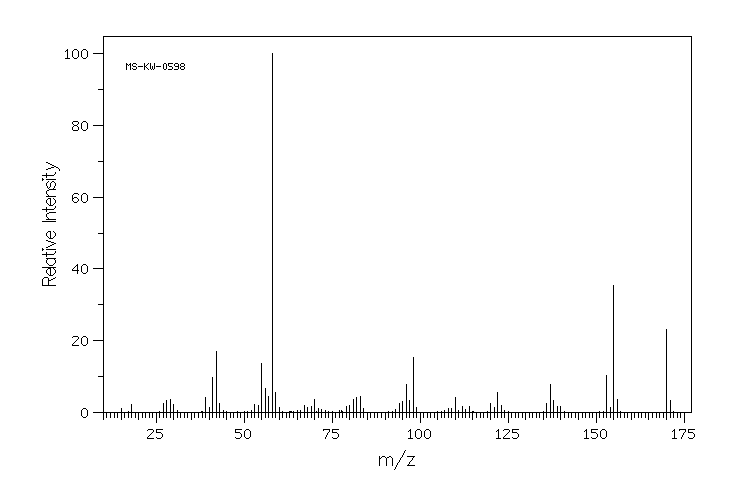
质谱MS
-
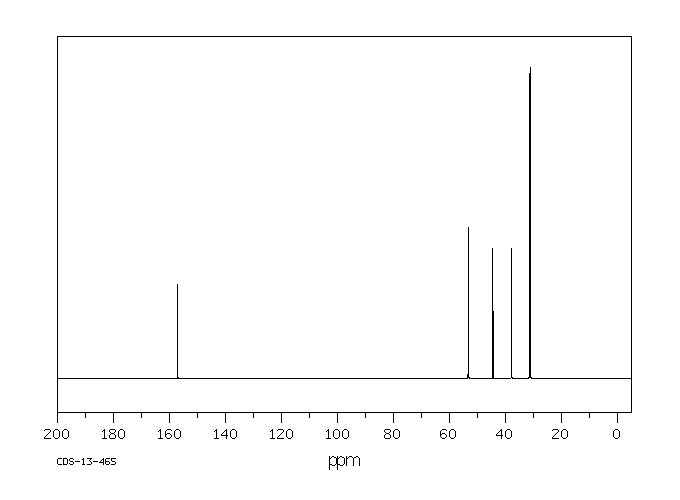
碳谱13CNMR
-
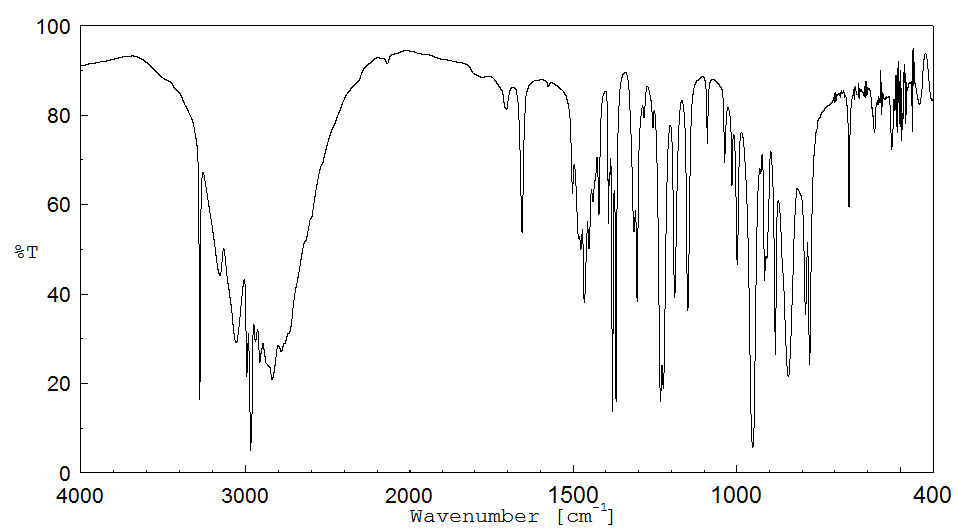
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-甲基哌啶盐酸盐;
(R)-2-苄基哌啶-1-羧酸叔丁酯
((3S,4R)-3-氨基-4-羟基哌啶-1-基)(2-(1-(环丙基甲基)-1H-吲哚-2-基)-7-甲氧基-1-甲基-1H-苯并[d]咪唑-5-基)甲酮盐酸盐
高氯酸哌啶
高托品酮肟
马来酸帕罗西汀
颜料红48:4
顺式3-氟哌啶-4-醇盐酸盐
顺式2,6-二甲基哌啶-4-酮
顺式1-苄基-4-甲基-3-甲氨基-哌啶
顺式-叔丁基4-羟基-3-甲基哌啶-1-羧酸酯
顺式-6-甲基-哌啶-1,3-二甲酸1-叔丁酯
顺式-5-(三氟甲基)哌啶-3-羧酸甲酯盐酸盐
顺式-4-叔丁基-2-甲基哌啶
顺式-4-Boc-氨基哌啶-3-甲酸甲酯
顺式-4-(氮杂环丁烷-1-基)-3-氟哌
顺式-3-顺式-4-氨基哌啶
顺式-3-甲氧基-4-氨基哌啶
顺式-3-BOC-3,7-二氮杂双环[4.2.0]辛烷
顺式-3-(1-吡咯烷基)环丁腈
顺式-3,5-哌啶二羧酸
顺式-3,4-二溴-3-甲基吡咯烷盐酸盐
顺式-2,6-二甲基-4-氧代哌啶-1-羧酸叔丁基酯
顺式-1-叔丁氧羰基-4-甲基氨基-3-羟基哌啶
顺式-1-boc-3,4-二氨基哌啶
顺式-1-(4-叔丁基环己基)-4-苯基-4-哌啶腈
顺式-1,3-二甲基-4-乙炔基-6-苯基-3,4-哌啶二醇
顺-4-(4-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-4-(2-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-3-氨基-4-氟哌啶-1-羧酸叔丁酯
顺-1-苄基-4-甲基哌啶-3-氨基酸甲酯盐酸盐
非莫西汀
雷芬那辛
雷拉地尔
阿维巴坦中间体4
阿格列汀杂质
阿尼利定盐酸盐 CII
阿尼利定
阿塔匹酮
阿哌沙班杂质BMS-591455
阿哌沙班杂质87
阿哌沙班杂质52
阿哌沙班杂质51
阿哌沙班杂质5
阿哌沙班杂质
阿哌沙班杂质
阿哌沙班-d3
阿哌沙班
阻聚剂701
间氨基谷氨酰胺