2,3-二苯基喹喔啉 1,4-二氧化物 | 5227-56-5
中文名称
2,3-二苯基喹喔啉 1,4-二氧化物
中文别名
2,3-二苯基喹喔啉1,4-二氧化物
英文名称
2,3-diphenylquinoxaline-N1,N4-dioxide
英文别名
2,3-diphenylquinoxaline N,N'-dioxide;2,3-diphenyl-quinoxaline 1,4-dioxide;2,3-Diphenyl-chinoxalin-1,4-dioxid;N,N'-Dioxo-2,3-diphenyl-chinoxalin;2,3-Diphenylchinoxalin-1,4-dioxid;2,3-Diphenylchinoxalin-N,N-dioxid;2,3-Diphenylquinoxaline 1,4-dioxide;4-oxido-2,3-diphenylquinoxalin-1-ium 1-oxide
CAS
5227-56-5
化学式
C20H14N2O2
mdl
——
分子量
314.343
InChiKey
NPTPZLXFFOWFIE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:24
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:46.4
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2933990090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2,3-diphenyl quinoxaline N-oxide 5227-55-4 C20H14N2O 298.344 2,3-二苯基喹喔啉 2,3-diphenylquinoxaline 1684-14-6 C20H14N2 282.345
反应信息
-
作为反应物:参考文献:名称:Jarrar,A.A. et al., Journal of Heterocyclic Chemistry, 1978, vol. 15, p. 177 - 179摘要:DOI:
-
作为产物:描述:参考文献:名称:一种新的高效路线为喹喔啉的形成Ñ -Oxides和N,N- ' -Dioxides使用HOF·CH 3 CN摘要:HOF·CH 3 CN,一个非常有效的氧转移剂容易从氟和含水乙腈制成,与各种喹喔啉衍生物反应,得到相应的单Ñ -oxides和尤其是N,N- “中在温和的非常良好的产率-dioxides条件和较短的反应时间。DOI:10.1021/jo0608016
文献信息
-
An unexpected course of the Meisenheimer reaction : aryl phosphates in the reaction of phosphoryl chloride with 2, 3-diphenylquinoxaline-N1-oxide.作者:J. Nasielski、S. Heilporn、R. Nasielski-Hinkens、F. Geerts-EvrardDOI:10.1016/s0040-4020(01)90308-4日期:1987.1Contrary to what is observed with other π-deficient heteroaromatic N-oxides, the reaction of 2, 3-diphenylquinoxaline-N1-oxide with OPCl3 gives only very poor yields of chlorinated quinoxalines. It is shown that the major product arises from an unprecedented attack by the nucleophilic oxygen atom of the reagent at a carbon atom of the homocycle of the O-phosphorylated N-oxide, leading ultimately to
-
Landquist; Stacey, Journal of the Chemical Society, 1953, p. 2822,2827作者:Landquist、StaceyDOI:——日期:——
-
Rosum; Lisowskaja, Zhurnal Obshchei Khimii, 1959, vol. 29, p. 228,233; engl. Ausg. S. 231, 235作者:Rosum、LisowskajaDOI:——日期:——
-
Glycosylated heterocyles emulsified with antifungal fraction of <i>Moringa oleifera</i> for potentiation of mycolytic activity作者:Manish Bhatia、Nayan Karnekar、Omkar Halingale、Snehal ArvindekarDOI:10.1080/14786419.2023.2222432日期:——
表征谱图
-
氢谱1HNMR
-
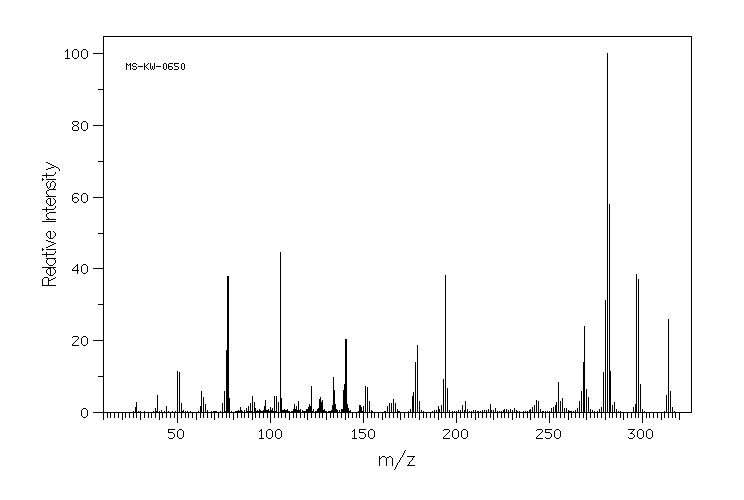
质谱MS
-
碳谱13CNMR
-
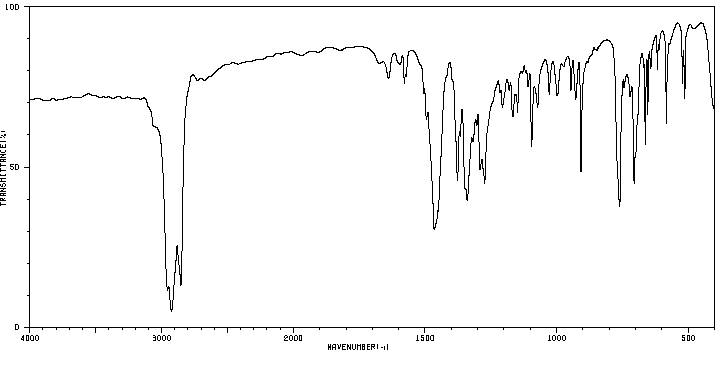
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E,Z)-他莫昔芬N-β-D-葡糖醛酸
(E/Z)-他莫昔芬-d5
(4S,5R)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S,5R,5''R)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(4R,5S)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4R,4''R,5S,5''S)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(1R,2R)-2-(二苯基膦基)-1,2-二苯基乙胺
鼓槌石斛素
黄子囊素
高黄绿酸
顺式白藜芦醇三甲醚
顺式白藜芦醇
顺式己烯雌酚
顺式-白藜芦醇3-O-beta-D-葡糖苷酸
顺式-桑皮苷A
顺式-曲札芪苷
顺式-二苯乙烯
顺式-beta-羟基他莫昔芬
顺式-a-羟基他莫昔芬
顺式-3,4',5-三甲氧基-3'-羟基二苯乙烯
顺式-1-(3-甲基-2-萘基)-2-(2-萘基)乙烯
顺式-1,2-双(三甲基硅氧基)-1,2-双(4-溴苯基)环丙烷
顺式-1,2-二苯基环丁烷
顺-均二苯乙烯硼酸二乙醇胺酯
顺-4-硝基二苯乙烯
顺-1-异丙基-2,3-二苯基氮丙啶
非洲李(PRUNUSAFRICANA)树皮提取物
阿非昔芬
阿里可拉唑
阿那曲唑二聚体
阿托伐他汀环氧四氢呋喃
阿托伐他汀环氧乙烷杂质
阿托伐他汀环(氟苯基)钠盐杂质
阿托伐他汀环(氟苯基)烯丙基酯
阿托伐他汀杂质D
阿托伐他汀杂质94
阿托伐他汀杂质7
阿托伐他汀杂质5
阿托伐他汀内酰胺钠盐杂质
阿托伐他汀中间体M4
阿奈库碘铵
锌(II)(苯甲醛)(四苯基卟啉)
银松素
铜酸盐(5-),[m-[2-[2-[1-[4-[2-[4-[[4-[[4-[2-[4-[4-[2-[2-(羧基-kO)苯基]二氮烯基-kN1]-4,5-二氢-3-甲基-5-(羰基-kO)-1H-吡唑-1-基]-2-硫代苯基]乙烯基]-3-硫代苯基]氨基]-6-(苯基氨基)-1,3,5-三嗪-2-基]氨基]-2-硫代苯基]乙烯基]-3-硫代
铒(III) 离子载体 I
铀,二(二苯基甲酮)四碘-
钾钠2,2'-[(E)-1,2-乙烯二基]二[5-({4-苯胺基-6-[(2-羟基乙基)氨基]-1,3,5-三嗪-2-基}氨基)苯磺酸酯](1:1:1)
钠{4-[氧代(苯基)乙酰基]苯基}甲烷磺酸酯
钠;[2-甲氧基-5-[2-(3,4,5-三甲氧基苯基)乙基]苯基]硫酸盐
钠4-氨基二苯乙烯-2-磺酸酯