1,2-dimethyldiaziridine | 6794-95-2
中文名称
——
中文别名
——
英文名称
1,2-dimethyldiaziridine
英文别名
N,N'-dimethylaziridine;1,2-dimethyl-diaziridine
CAS
6794-95-2;108602-72-8;279675-51-3
化学式
C3H8N2
mdl
——
分子量
72.1099
InChiKey
HHUSPFWAFUDXFF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):0.4
-
重原子数:5
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:6
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:描述:1,2-dimethyldiaziridine 、 丁炔二酸二乙酯 在 1-butyl-3-methylimidazolium Tetrafluoroborate 作用下, 反应 0.52h, 以34%的产率得到diethyl 1-[3-ethoxy-1-(ethoxycarbonyl)-3-oxoprop-1-enyl]-3-methyl-1,2,3,6-tetrahydropyrimidine-4,5-dicarboxylate参考文献:名称:1,2-二烷基和1,2,3-三烷基二氮丙啶的新反应:在离子液体中乙炔二羧酸二乙酯作用下的扩环摘要:在乙二烯二羧酸二乙酯作用于离子中的1,2-二-和1,2,3-三烷基二氮丙啶的作用下,发现了二氮丙啶环扩环的新反应,从而生成1,2,3,6-四氢嘧啶-4,5-二羧酸二乙酯衍生物液体。J.杂环化学,(2009)。DOI:10.1002/jhet.204
-
作为产物:描述:1,3,5-三甲基己羟基-1,3,5-三嗪 、 N-氯甲胺 在 水 、 potassium carbonate 作用下, 以 氯仿 为溶剂, 以45%的产率得到1,2-dimethyldiaziridine参考文献:名称:Syntheses of 1,2-di- and 1,2,3-trialkyldiaziridines摘要:The reactions of 1,3,5-trialkylhexahydro-1,3,5-triazines with N-chloroalkylamines resulted in 1,2-dialkyldiaziridines, whereas the reactions of N-chloroalkylamines or N,N-dichloroalkylamines with an excess of primary aliphatic amines gave 1,2,3-trialkyldiaziridines.DOI:10.1070/mc2005v015n03abeh002107
文献信息
-
New method for the synthesis and the mechanism of formation of 1,2-di-and 1,2,3-trialkyldiaziridines作者:V. V. Kuznetsov、V. B. Ovchinnikova、V. P. Ananikov、N. N. MakhovaDOI:10.1007/s11172-006-0548-9日期:2006.11A new simple approach to the synthesis of 1,2-di-and 1,2,3-trialkyldiaziridines has been developed based on direct chlorination of a mixture of the corresponding aldehyde and an excess of primary aliphatic amine in water. The mechanism of this reaction is proposed and confirmed by quantum chemical calculations at the density functional theory level.
-
Kinetic and quantum chemical studies of the mechanism of formation of 1,2-dialkyldiaziridines作者:V. V. Kuznetsov、V. V. Seregin、A. A. Laptev、D. V. Khakimov、T. S. Pivina、A. P. Simakova、M. D. Vedenyapina、A. A. Vedenyapin、N. N. MakhovaDOI:10.1007/s11172-012-0152-0日期:2012.6spectrometry. The rate constants of particular steps of the reaction were estimated starting from the possibility of formation of the precursor of 1,2-dialkyldiaziridine, N-halogenaminal, due to the amination of the intermediate iminium cation along with the parallel halogenation of the intermediate gem-diamine. The quantum chemical calculations (DFT, B3LYP, 6–31++G(d,p) and 3–21G basis sets) were performed
-
The role of pH in the synthesis of diaziridines作者:V. V. Kuznetsov、N. N. Makhova、L. I. Khmel'nitskiiDOI:10.1007/bf02495943日期:1997.7In the synthesis of diaziridines from amines, carbonyl compounds, and NaOCl in water, the yields of 1,2-dialkyldiaziridines and of 1,2,3-trisubstituted diaziridines prepared from amines with electron-withdrawing substituents in the side chain are less sensitive to changes in pH than the yields of 1,2,3-trialkyldiaziridines with simple alkyl substituents. The formation of 1,5-diazabicyclo[3.1.0]hexanes
-
KUZNETSOV, V. V.;MAXOVA, N. N.;XMELNITSKIJ, L. I., IZV. AN CCCP. CEP. XIM.,(1989) N, S. 2090-2094作者:KUZNETSOV, V. V.、MAXOVA, N. N.、XMELNITSKIJ, L. I.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
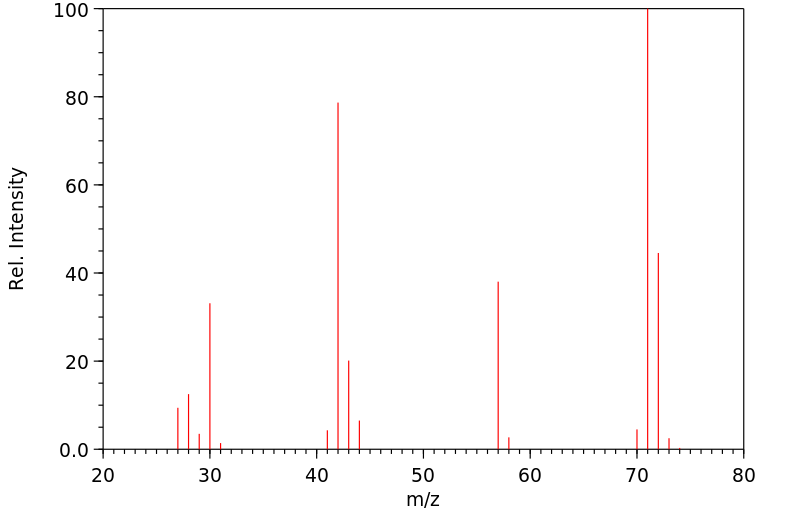
质谱MS
-
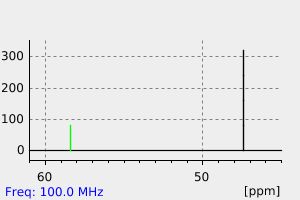
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷