9-Benzylidene-9H-xanthene | 27980-52-5
中文名称
——
中文别名
——
英文名称
9-Benzylidene-9H-xanthene
英文别名
9-Benzyliden-xanthen;9-benzylidene xanthene;9-Benzylidenexanthene
CAS
27980-52-5
化学式
C20H14O
mdl
——
分子量
270.331
InChiKey
GGXXRTLDWMBVQG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:114-115 °C
-
沸点:390.9±32.0 °C(Predicted)
-
密度:1.201±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):5
-
重原子数:21
-
可旋转键数:1
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
上下游信息
反应信息
-
作为反应物:描述:9-Benzylidene-9H-xanthene 在 1,4-二氧六环 、 lithium aluminium tetrahydride 、 乙醚 作用下, 生成 9-(4-chlorobenzylidene)-9H-xanthene参考文献:名称:Bergmann et al., Bulletin de la Societe Chimique de France, 1951, p. 669,680摘要:DOI:
-
作为产物:描述:参考文献:名称:9-(α-羟烷基)呫吨的合成和 Wagner-Meerwein 重排为 10-取代的二苯并[b,f]oxepins:范围、限制和从头算计算摘要:通过以下方法以良好的收率制备了一系列 9-(羟基)烷基氧杂蒽 5:(a) 将 9-锂氧杂蒽添加到官能化乙醛中,或者通过一种新方法,(b) 将碳负离子添加到氧杂蒽-9-甲醛中。为后者找到了一种实用且有效的合成方法。在酸性催化下,大多数加成产物经历 Wagner-Meerwein 重排,得到相应的 10-取代二苯并[b,f]oxepin 6 或 xanthenylid-9-ene β-消除产物 7。第一个 Wagner-Meerwein报道了高苄基氰醇的重排。二苯并[b,f]oxepins是神经活性物质的潜在前体。为了使产物分布合理化,并探索新重排的范围,在选定的情况下对产物和过渡态进行了从头算量子力学计算。DOI:10.1055/s-2005-872110
文献信息
-
Iodine-catalyzed Transformation of Aryl-substituted Alcohols Under Solvent-free and Highly Concentrated Reaction Conditions作者:Marjan Jereb、Dejan VražičDOI:10.17344/acsi.2017.3841日期:2017.12.15transformations of alcohols under solvent-free reaction conditions (SFRC) and under highly concentrated reaction conditions (HCRC) in the presence of various solvents were studied in order to gain insight into the behavior of the reaction intermediates under these conditions. Dimerization, dehydration and substitution were the three types of transformations observed with benzylic alcohols. Dimerization
-
Pd-Catalyzed Autotandem Reactions with <i>N</i>-Tosylhydrazones. Synthesis of Condensed Carbo- and Heterocycles by Formation of a C–C Single Bond and a C═C Double Bond on the Same Carbon Atom作者:Miguel Paraja、Carlos ValdésDOI:10.1021/acs.orglett.7b00613日期:2017.4.21A new Pd-catalyzed autotandem reaction is introduced that consists of the cross-coupling of a benzyl bromide with a N-tosylhydrazone followed by an intramolecular Heck reaction with an aryl bromide. During the process, a single and a double C–C bond are formed on the same carbon atom. Two different arrangements for the reactive functional groups are possible, rendering great flexibility to the transformation
-
Einfache Synthesen von 1,2-Diaryl- und Triarylethenen mit trägergebundenen Fluoridbasen作者:D. Hellwinkel、K. Göke、R. KarleDOI:10.1055/s-1994-25617日期:——Facile Syntheses of 1,2-Diaryl- and Triarylethenes with Supported Fluoride Bases Differently substituted arenecarbaldehydes, mainly benzaldehydes, can be very efficiently condensed with a wide variety of methylbenzenes having an electron-withdrawing group in the para-position, as well as with fluorenes, xanthene, cyclopentadienes and indenes by using a standardized KF- or CsF-Al2O3 base system in dimethylformamide.
-
Reaction of Olefins with Malonamide in the Presence of Manganese(III) Acetate. Formation of α,β-Unsaturated γ-Lactones and γ-Lactams作者:Hiroshi NishinoDOI:10.1246/bcsj.58.217日期:1985.1The reaction of olefins with malonamide in the presence of manganese(III) acetate gave 2-buten-4-olides and/or 1H-pyrrol-2(5H)-ones in one-step, short reaction time, and moderate yields. The product distribution can be accounted for in terms of the stabilization of the intermediate carbocation in the oxidation process. The synthetic application and limitation, and the oxidation mechanism for the formations
-
One-Pot Synthesis of Spirofuran and Benzocycloalka[1,2-<i>b</i>]furan Derivatives Using Manganese(III)-Mediated Oxidative Radical Cyclization作者:Hiroshi Nishino、Reika FujinoDOI:10.1055/s-2005-861807日期:——obtained by the oxidation of methylenebenzocycloalkanes with manganese(III) acetate in the presence of 1,3-dicarbonyl compounds. A similar oxidation of the benzocycloalkene derivatives produced the functionalized benzocycloalka[1,2-b]furans in good yields. However, l-benzyl-3,4-dihydronaphthalene gave both the corresponding spirofuran and benzocyclohexa[1,2-b]furan under the same oxidation conditions.
表征谱图
-
氢谱1HNMR
-
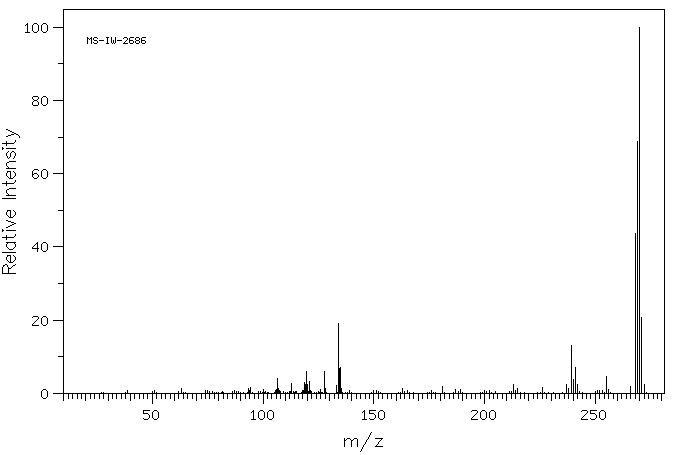
质谱MS
-
碳谱13CNMR
-
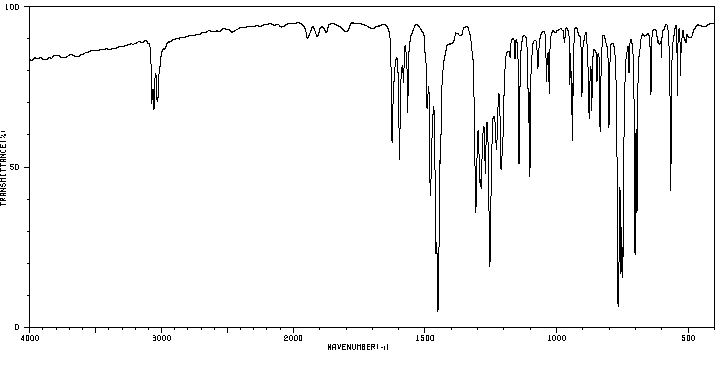
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-8-氟苯并二氢吡喃-4-胺
(2S)-6-氟-3,4-二氢-4-氧代-2H-1-苯并吡喃-2-甲酸
(2R)-6-氟-3,4-二氢-4-氧代-2H-1-苯并吡喃-2-甲酸
龙胆根素
龙胆SHAN酮
齿阿米素
黑色素-1
黄天精
麥角黃酮酸
鲍迪木醌
高邻苯二甲酸酐
高芒果苷
高氯酸罗丹明640
马佐卡林
香豆素-340
香豆素 339
食用色素红色105号
颜料红90:1铝色淀[CI45380:3]
颜料红172铝色淀[CI45430:1]
雏菊叶龙胆酮
降阿赛里奥; 1,3,6,7-四羟基氧杂蒽酮
阿米醇
阿米凯林
阿米凯林
阿扎那托
阿巴哌酮
阿尼地坦
阿尼地坦
阿匹氯铵
锌离子荧光探针-4
锆(2+)二[2-(2,4,5,7-四溴-3,6-二羟基氧杂蒽-9-基)-3,4,5,6-四氯苯甲酸酯]
铁力木呫吨酮-B
铁力木吨酮A
钠6'-羟基-5-[2-[4-(2-甲基-3-氧代-7H-咪唑并[1,2-d]吡嗪-6-基)苯氧基]乙基硫代氨基甲酰氨基]-3-氧代螺[2-苯并呋喃-1,9'-氧杂蒽]-3'-醇
钠4-{[6'-(二乙基氨基)-3'-羟基-3-氧代-3H-螺[2-苯并呋喃-1,9'-氧杂蒽]-2'-基]偶氮}-3-羟基-1-萘磺酸酯
钠2-(2,7-二氯-9H-氧杂蒽-9-基)苯甲酸酯
钙黄绿素乙酰甲酯
钙荧光探针Fluo-8,AM
钙荧光探针Fluo-4,AM
钙离子荧光探针
钙柑子
采木(HAEMATOXYLONCAMPECHIANUM)木质提取物
酸性媒介桃红3BM
邻苯三酚红
远志山酮III
转移核糖核酸(面包酵母)
赤藓红B异硫氰酸酯异构体II
赤藓红
诺大麻
西伯尔链接剂