2-丁基四氢噻吩 | 1613-49-6
中文名称
2-丁基四氢噻吩
中文别名
——
英文名称
2-Butyl-thiophan
英文别名
2-Butyltetrahydrothiophene;2-butylthiolane
CAS
1613-49-6
化学式
C8H16S
mdl
——
分子量
144.281
InChiKey
NBPQJOLVXAKDFK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:201.5-202.0 °C(Press: 753 Torr)
-
密度:0.9221 g/cm3
-
保留指数:1131;1133;1133;1133;1133
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:9
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:25.3
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2934999090
SDS
反应信息
-
作为反应物:参考文献:名称:VOLYNSKIJ N. P.; SHCHERBAKOVA L. P., IZV. AN CCCP. CEP. XIM., 1979, HO 5, 1080-1085摘要:DOI:
-
作为产物:参考文献:名称:CLARK, PETER D.;DOWLING, NORMAN I.;LESAGE, KEVIN L.;HYNE, JAMES B., FUEL, 66,(1987) N 12, 1699-1702摘要:DOI:
文献信息
-
Regioselective indium(<scp>iii</scp>) trifluoromethanesulfonate-catalyzed hydrothiolation of non-activated olefins作者:Michel Weïwer、Lydie Coulombel、Elisabet DuñachDOI:10.1039/b513946e日期:——Indium(III) trifluoromethanesulfonate was found to be an excellent catalyst for the highly regioselective intra- and intermolecular addition of thiols to non-activated olefins and could be recycled and reused without loss of activity.
-
Stereospecific cyclodehydration of 1,4-sulfanylalcohols to thiolanes: mechanistic insights作者:Jean-Jacques Filippi、Elisabet Duñach、Xavier Fernandez、Uwe J. MeierhenrichDOI:10.1016/j.tet.2008.07.088日期:2008.10A series of thiolanes were prepared by cyclodehydration of sulfanylalcohols in the presence of catalytic amounts of p-toluenesulfonic acid or by using K10 clay. The sulfur heterocycles were synthesised in good to excellent yields using either a conventional Dean–Stark method or microwave irradiation under solvent-free conditions. The reaction could be performed regio- and stereoselectively and its
-
[EN] INTEGRATED PROCESS FOR PRODUCING CYCLIC ACETALS AND OXYMETHYLENE POLYMERS<br/>[FR] PROCÉDÉ INTÉGRÉ DE PRODUCTION D'ACÉTALS CYCLIQUES ET DE POLYMÈRES D'OXYMÉTHYLÈNE申请人:TICONA GMBH公开号:WO2013076288A1公开(公告)日:2013-05-30A process for producing cyclic acetals is described. A formaldehyde source is contacted with an aprotic compound in the presence of a catalyst to produce the cyclic acetals. The aprotic compound can increase conversion rates and/or efficiency. In one embodiment, the formaldehyde source is obtained from methanol. In particular, methanol can be converted into formaldehyde which is then converted into a cyclic acetal. In one embodiment, the cyclic acetal can then be used to produce oxymethylene polymers.
-
[EN] PROCESS FOR PRODUCING A CYCLIC ACETAL IN A HETEROGENEOUS REACTION SYSTEM<br/>[FR] PROCÉDÉ DE PRODUCTION D'UN ACÉTAL CYCLIQUE DANS UN SYSTÈME RÉACTIONNEL HÉTÉROGÈNE申请人:TICONA GMBH公开号:WO2013076290A1公开(公告)日:2013-05-30A process for producing a cyclic acetal is disclosed. According to the process, a formaldehyde source is combined with an aprotic compound and contacted with a heterogeneous catalyst which causes the formaldehyde source to convert into a cyclic acetal such as trioxane. The catalyst, for instance, may comprise a solid catalyst such as an ion exchange resin. In one embodiment, the process is used for converting anhydrous formaldehyde gas to trioxane. The anhydrous formaldehyde gas may be produced form an aqueous formaldehyde solution by an extractive distillation.
-
Process for the manufacture of high purity linear c4+ alkyl mercaptans申请人:ELF ATOCHEM NORTH AMERICA, INC.公开号:EP0706998A1公开(公告)日:1996-04-17Linear C₄+ alkyl mercaptans of high purity are prepared by the reaction of a di-linear(C₄+) alkyl sulfide with hydrogen sulfide at elevated temperature in the presence of an alumina material selected from gamma/eta alumina or gamma/eta alumina impregnated with up to 20% w/w of titania or rhenium oxide.
表征谱图
-
氢谱1HNMR
-
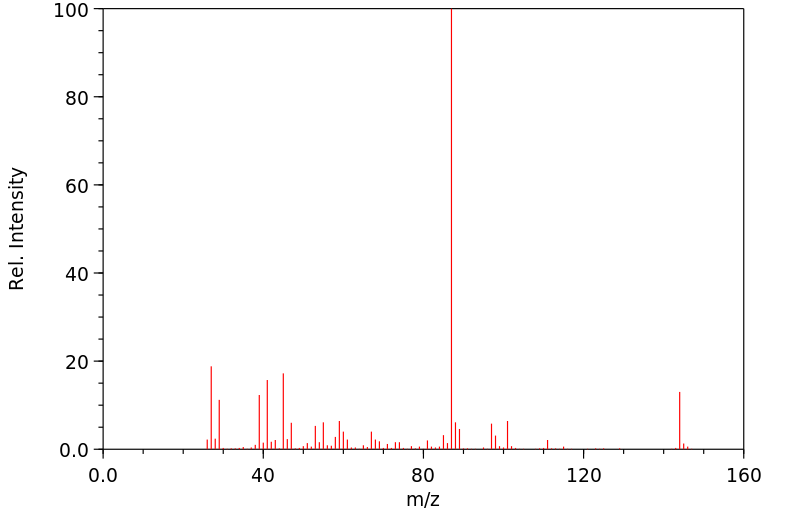
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
苯甲酸,4-(1,3-二噁烷-2-基)-
红色基KL
甲基四氢-2-噻吩羧酸酯
甲基4-氧代四氢-2-噻吩羧酸酯
环丁砜
烯丙基-(3-甲基-1,1-二氧代-四氢-1lambda*6*-噻吩-3-基)-胺
氯(四氢噻吩)金(I)
四甲基亚砜
四氢噻吩二醇
四氢噻吩-3-酮
四氢噻吩-3-羧酸-1,1-二氧
四氢噻吩-2,5-二酮
四氢噻吩-1,1-二亚基二胺
四氢噻吩
四氢-噻吩-3-醇
四氢-N-甲基-N-亚硝基-3-噻吩胺1,1-二氧化物
四氢-3-噻吩羧酸甲酯
四氢-3-噻吩羧酸
四氢-3-噻吩磺酰氯 1,1-二氧化物
四氢-3-噻吩硫醇1,1-二氧化物
四氢-3-噻吩甲酰氯1,1-二氧化物
四氢-3-噻吩甲腈1,1-二氧化物
四氢-3-噻吩基甲基丙烯酸酯
四氢-3,4-噻吩二胺1,1-二氧化物
四氢-2-噻吩羧酸
四亚甲基-D8砜
噻吩,四氢-2,2,5,5-四甲基-
反式-3-辛基亚磺酰基-4-羟基四氢噻吩1,1-二氧化物
八氟四氢噻吩 1,1-二氧化物
全氟四氢噻吩
二甲基砜茂烷
二氢-5,5-二甲基噻吩-3(2H)-酮
二氢-2-甲基-3(2H)-噻吩酮
乙基四氢-3-噻吩羧酸酯
乙基(5Z)-5-(羟基亚胺)-4-氧代-4,5-二氢-3-噻吩羧酸酯
乙基(4E)-4-(羟基亚胺)四氢-3-噻吩羧酸酯
Γ--硫代丁内酯
beta-乙基-beta-甲基-硫代丁内酯
alpha-乙基,alpha-甲基-硫代丁内酯
[[[(四氢噻吩1,1-二氧化物)-3-基]亚氨基]二(亚甲基)]二膦酸
[(1,1-二氧代四氢噻吩-3-基)氨基]二硫代甲酸
[(1,1-二氧代四氢-3-噻吩基)甲基]胺
[(1,1-二氧代-3-四氢噻吩基)氨基]二硫代甲酸钾盐
REL-(3AS,6AS)-六氢-2H-噻吩并[2,3-C]吡咯1,1-二氧化物盐酸盐
N-(四氢呋喃-2-基甲基)-N-四氢噻吩-3-基胺
N-烯丙基四氢-3-噻吩胺1,1-二氧化物
N-丁基-N-(1,1-二氧代四氢噻吩-3-基)胺盐酸盐
N-(1,1-二氧代四氢噻吩-3-基)乙酰胺
N'-(1,1-二氧代-四氢噻吩-3-基)-N,N-二甲基-乙烷-1,2-二胺
7-硫杂双环[2.2.1]庚-5-烯-2-羧酸