1-(morpholinyl)phenazine | 22316-92-3
中文名称
——
中文别名
——
英文名称
1-(morpholinyl)phenazine
英文别名
4-(phenazin-1-yl)morpholine;1-Morpholino-phenazin;1-morpholin-4-yl-phenazine;1-Morpholinophenazine;4-phenazin-1-ylmorpholine
CAS
22316-92-3
化学式
C16H15N3O
mdl
——
分子量
265.315
InChiKey
PFXKNWOHAOXEFO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:491.1±30.0 °C(Predicted)
-
密度:1.279±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:20
-
可旋转键数:1
-
环数:4.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:38.2
-
氢给体数:0
-
氢受体数:4
反应信息
-
作为产物:描述:1-chlorophenazine 5-oxide 在 copper dichloride 作用下, 生成 1-(morpholinyl)phenazine参考文献:名称:Synthesis of 1- and 2-Morpholinophenazine Derivatives摘要:已经通过各种卤代苯并呲嗪衍生物与吗啉的反应制备了一些新的吗啉苯并呲嗪。研究发现,在卤代苯并呲嗪-N-氧化物中,用吗啉替换卤素原子时伴随着氧化物基团的去除,并且在这些反应中没有形成吗啉苯并呲嗪-N-氧化物。DOI:10.1246/bcsj.42.506
文献信息
-
An umpolung strategy for rapid access to thermally activated delayed fluorescence (TADF) materials based on phenazine作者:Huaxing Zhang、Qiang Guo、Hu Cheng、Chunhao Ran、Di Wu、Jingbo LanDOI:10.1039/d1cc06705b日期:——radical nucleophilic addition/rearrangement of 2-aryl diazaboroles has been accomplished for the first time to construct phenazine structures. This protocol is an umpolung strategy based on the classical electrophilic mechanism, and therefore, a reversed regioselectivity was observed, which provides an opportunity to prepare sterically hindered phenazines. The resulting thermally activated delayed fluorescence
-
Synthesis of 1-substituted phenazines as novel antichlamydial agents作者:Xiao-Feng Bao、Yi-Xin Zhu、Wen-Xia Xie、Zi-Yi Liu、Li Zhu、He Jiang、Yu ZhaoDOI:10.1080/10286020.2021.1982909日期:2022.9.2Abstract A novel series of 1-substituted phenazines 4a-4l were designed and synthesized via Palladium-catalyzed reactions from 1-phenazine trifluoromethanesulfonate. These phenazines showed antichlamydial activity with IC50 values from 1 to 10 μM. Among them, compounds 4c and 4i exhibited the best antichlamydial activity with IC50 values from 2.06 to 2.74 μM without apparent cytotoxicity to host cells
表征谱图
-
氢谱1HNMR
-
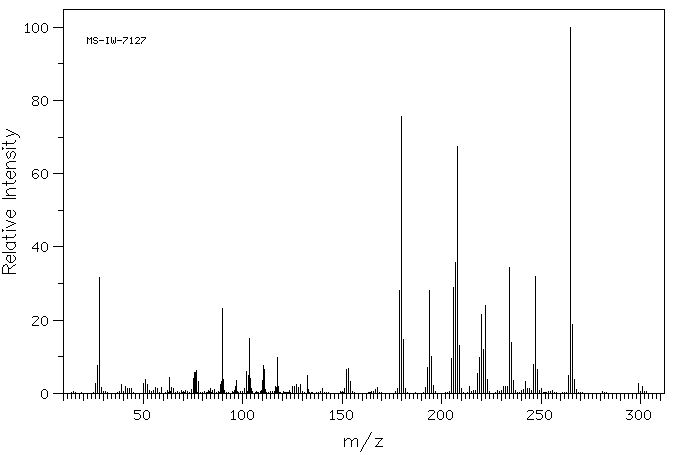
质谱MS
-
碳谱13CNMR
-
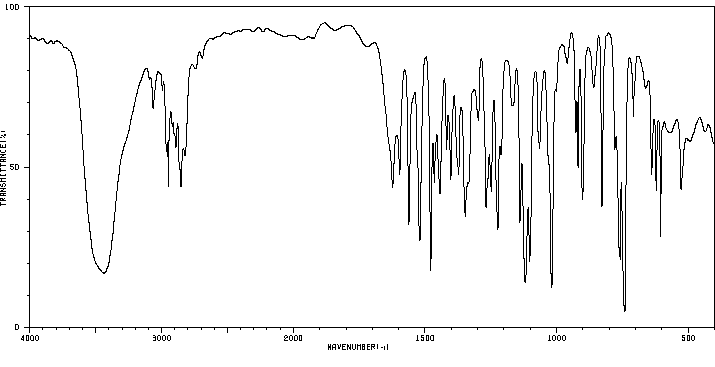
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮