4,6-二甲基-1-苯并呋喃-2,3-二酮 | 31297-31-1
中文名称
4,6-二甲基-1-苯并呋喃-2,3-二酮
中文别名
——
英文名称
4,6-dimethylbenzofuran-2,3-dione
英文别名
4,6-Dimethyl-benzofuran-2,3-dion;4,6-dimethyl-1-benzofuran-2,3-dione
CAS
31297-31-1
化学式
C10H8O3
mdl
——
分子量
176.172
InChiKey
YXLOMQUUGPIAIU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:144 °C(Solv: ligroine (8032-32-4))
-
沸点:323.5±52.0 °C(Predicted)
-
密度:1.303±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:13
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:43.4
-
氢给体数:0
-
氢受体数:3
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4,6-二甲基-1-苯并呋喃-3(2H)-酮 4,6-dimethylbenzofuran-3(2H)-one 20895-44-7 C10H10O2 162.188
反应信息
-
作为反应物:描述:4,6-二甲基-1-苯并呋喃-2,3-二酮 以40%的产率得到参考文献:名称:FRIEDRICHSEN W., J. LIEBIGS ANN. CHEM.
, 1975, NO 9, 1545-1562 摘要:DOI: -
作为产物:描述:4,6-二甲基-1-苯并呋喃-3(2H)-酮 在 air 、 乙醚 、 sodium ethanolate 、 Petroleum ether 作用下, 生成 4,6-二甲基-1-苯并呋喃-2,3-二酮参考文献:名称:607.螺环化合物。第一部分:与3:2'-二氧戊酸相关的化合物的制备摘要:DOI:10.1039/jr9570003112
文献信息
-
Nickel‐Catalyzed Decarbonylative Cycloaddition of Benzofuran‐2,3‐diones with Alkynes to Flavones作者:Yu‐Yang Zhang、Han Li、Xiaoding Jiang、Chitreddy V Subba Reddy、Hao Liang、Yaqi Zhang、Rihui Cao、Raymond Wai‐Yin Sun、Man Kin Tse、Liqin QiuDOI:10.1002/adsc.202101241日期:2022.2the Nickel-catalyzed decarbonylative cycloaddition of benzofuran-2,3-diones with alkynes was established, and a variety of functional flavones were synthesized in 65–99% yields. Terminal alkynes with substituted phenyl groups and internal alkynes such as aryl acyl acetylenes and diphenylacetylenes are suitable for this reaction. The effects of bases on the reactions of different types of alkyne substrates
-
一种黄酮类化合物的制备方法申请人:中山大学公开号:CN112939917B公开(公告)日:2023-08-22
-
Ruthenium-Catalyzed Carbonylative Cycloaddition of α-Keto Lactones with Alkenes or Alkynes: The Participation of an Ester-Carbonyl Group in Cycloaddition Reactions as the Two-Atom Assembling Unit作者:Naoto Chatani、Katsuya Amako、Mamoru Tobisu、Taku Asaumi、Yoshiya Fukumoto、Shinji MuraiDOI:10.1021/jo0267495日期:2003.2.1reaction of benzofuran-2,3-dione derivatives 1 with CO and alkenes (or alkynes) results in a carbonylative [2+2+1] cycloaddition in which the ester-carbonyl group is incorporated into a two-atom assembling unit to give spirolactone derivatives 2. This reaction provides the first example of an ester-carbonyl group participating in a carbonylative cycloaddition reaction.
-
An efficient diastereoselective synthesis of spiro pyrido[2,1-b][1,3]oxazines via a novel pyridine-based three-component reaction作者:Abbas Ali Esmaeili、Hamid Vesalipoor、Rahele Hosseinabadi、Alireza Fakhari Zavareh、Mohammad Ali Naseri、Ebrahim GhiamatiDOI:10.1016/j.tetlet.2011.07.039日期:2011.9The zwitterions generated from pyridine and dialkyl acetylenedicarboxylate (DAAD) reacted with benzofuran-2,3-diones to form highly substituted spiro pyrido[2,1-b][1,3]oxazines in good to high yields without using a catalyst.
-
A novel reaction of the ‘Huisgen zwitterion’ with benzofuran-2,3-diones: an efficient strategy for the synthesis of spirocyclic benzofuran-2-one derivatives作者:Ze-Dong Wang、Nan Dong、Feng Wang、Xin Li、Jin-Pei ChengDOI:10.1016/j.tetlet.2013.07.129日期:2013.10nitrogen-based nucleophile generated from azodicarboxylate and triphenylphosphine displayed good reactivity toward benzofuran-2,3-diones to generate a variety of spirocyclic benzofuran-2-one derivatives. The reactions accommodate a number of benzofuran-2,3-diones and different dialkylazodicarboxylates to give the enriched functionalized 3-spirooxadiazole benzofuran-2-ones with moderate to good yields (up
表征谱图
-
氢谱1HNMR
-
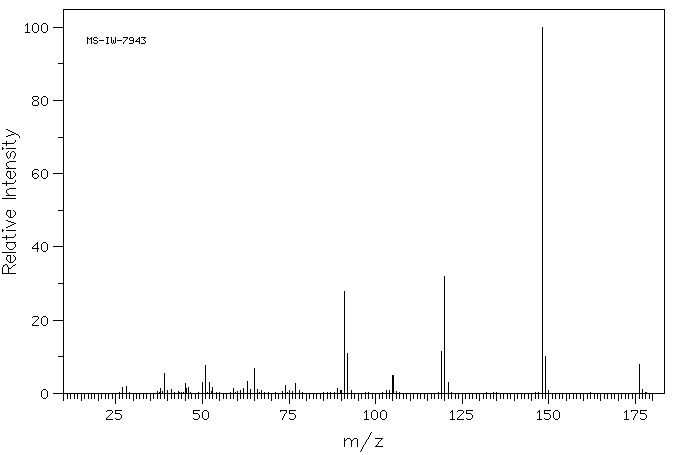
质谱MS
-
碳谱13CNMR
-
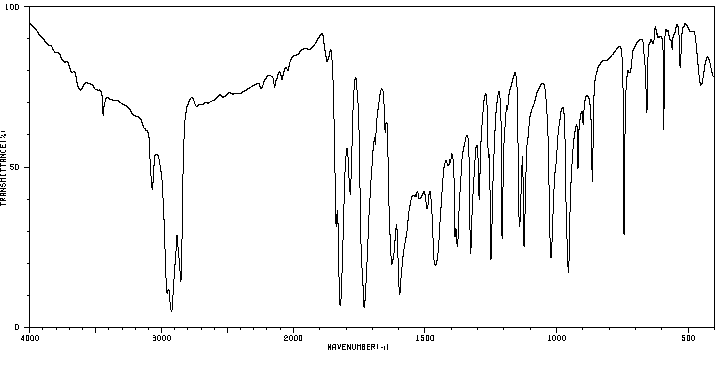
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
()-2-(5-甲基-2-氧代苯并呋喃-3(2)-亚乙基)乙酸乙酯
顺式-1-((2-(5-氯-2-苯并呋喃基)-4-甲基-1,3-二氧戊环-2-基)甲基)-1H-1,2,4-三唑
顺式-1-((2-(5,7-二氯-2-苯并呋喃基)-4-乙基-1,3-二氧戊环-2-基)甲基)-1H-咪唑
顺式-1-((2-(2-苯并呋喃基)-4-乙基-1,3-二氧戊环-2-基)甲基)-1H-1,2,4-三唑
霉酚酸酯杂质B
雷美替胺杂质3
雷美替胺杂质22
雷美替胺杂质
间甲酚紫
间甲基苯基(苯并呋喃-2-基)甲醇
长管假茉莉素C
钠1,4-二[(2-乙基己基)氧基]-1,4-二氧代-2-丁烷磺酸酯-3,3-二(4-羟基苯基)-2-苯并呋喃-1(3H)-酮(1:1:1)
金霉素
酪氨酸,b-羰基-
酞酸酐-d4
酚酞二丁酸酯
酚酞
酚红钠
酚红
邻苯二甲酸酐与马来酸酐,甘氨酰蜡素和二乙二醇的聚合物
邻苯二甲酸酐与己二醇的聚合物
邻苯二甲酸酐与三甘醇异壬醇的聚合物
邻苯二甲酸酐与2-乙基-2-羟甲基-1,3-丙二醇和2,5-呋喃二酮的聚合物
邻苯二甲酸酐与2-乙基-2-羟甲基-1,3-丙二醇、2,5-呋喃二酮和2-乙基己酸苯甲酸酯的聚合物
邻苯二甲酸酐-13C6
邻苯二甲酸酐-4-硼酸频哪醇酯
邻苯二甲酸酐,马来酸,二乙二醇,新戊二醇聚合物
邻甲酚酞二庚酸酯
邻甲酚酞二己酸酯
邻甲酚酞
贝康唑
表灰黄霉素
螺佐呋酮
螺[苯并呋喃-3(2H),4-哌啶]
螺[异苯并呋喃-1(3H),4’-哌啶]-3-酮
螺[异苯并呋喃-1(3H),4'-哌啶]-3-酮盐酸盐
螺[异苯并呋喃-1(3H),3’-吡咯烷]-3-酮
螺[1-苯并呋喃-2,1'-环丙烷]-3-酮
薄荷内酯
萘并[2,3-b]呋喃-8(4H)-酮,4a,5,6,7,8a,9-六氢-,顺-
莫罗卡尼
荨麻叶泽兰酮
荧光胺
苯酞-3-乙酸
苯酚,2-[3-(2-苯并呋喃基)-5,6-二氢-1,2,4-三唑并[3,4-b][1,3,4]噻二唑-6-基]-
苯酐二乙二醇共聚物
苯酐
苯甲酸,2-[(1,3-二羰基丁基)氨基]-,甲基酯
苯甲酸,2,2-二(羟甲基)丙烷-1,3-二醇,异苯并呋喃-1,3-二酮
苯甲酰氯化,3-甲氧基-4-甲基-