1',3'-dihydro-3',3'-dimethyl-6-nitro-1'-octadecyl-8-(docosanoyloxymethyl)spiro[2H-1-benzopyran-2,2'-(2H)-indole] | 103825-02-1
中文名称
——
中文别名
——
英文名称
1',3'-dihydro-3',3'-dimethyl-6-nitro-1'-octadecyl-8-(docosanoyloxymethyl)spiro[2H-1-benzopyran-2,2'-(2H)-indole]
英文别名
(3',3'-Dimethyl-6-nitro-1'-octadecylspiro[chromene-2,2'-indole]-8-yl)methyl docosanoate
CAS
103825-02-1
化学式
C59H96N2O5
mdl
——
分子量
913.422
InChiKey
SYNAMBCINCGUBN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):23.9
-
重原子数:66
-
可旋转键数:40
-
环数:4.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:84.6
-
氢给体数:0
-
氢受体数:6
反应信息
-
作为反应物:描述:1',3'-dihydro-3',3'-dimethyl-6-nitro-1'-octadecyl-8-(docosanoyloxymethyl)spiro[2H-1-benzopyran-2,2'-(2H)-indole] 在 双十八烷基二甲基溴化铵 作用下, 以 水 为溶剂, 反应 0.08h, 生成 [5-[2-(3,3-Dimethyl-1-octadecylindol-2-ylidene)ethylidene]-3-nitro-6-oxocyclohexa-1,3-dien-1-yl]methyl docosanoate参考文献:名称:双层膜中光致变色螺吡喃的头对尾和并排聚集体的形成摘要:DOI:10.1021/j100372a077
-
作为产物:描述:[5-[2-(3,3-Dimethyl-1-octadecylindol-2-ylidene)ethylidene]-3-nitro-6-oxocyclohexa-1,3-dien-1-yl]methyl docosanoate 以 various solvent(s) 为溶剂, 生成 1',3'-dihydro-3',3'-dimethyl-6-nitro-1'-octadecyl-8-(docosanoyloxymethyl)spiro[2H-1-benzopyran-2,2'-(2H)-indole]参考文献:名称:螺吡喃的光致变色在有组织的分子组装中。双层-粘土基质中光金属花青的J和H聚集体的形成摘要:一系列具有不同烷基长度的1'-烷基-3',3'-二甲基-6-硝基-8-烷酰氧基甲基螺(2 H -1-苯并吡喃-2,2'-二氢吲哚)衍生物的光致变色已经在十二烷基二甲基氯化铵-粘土基质中研究了链。当辐照含有适当长度的烷基链的SP时,会形成稳定的H-(平行型)和J(头对尾型)光合花青素聚集体。DOI:10.1039/c39910000532
文献信息
-
Photochromic compounds useful in Langmuir-Blodgett films申请人:BRITISH TECHNOLOGY GROUP LIMITED公开号:EP0391631A1公开(公告)日:1990-10-10Compounds of formula (I); in which A represents certain pyridinium, quinolinium, isoquinolinium or pyrimidinium groups unsubstituted or substituted by F, Cl, Br, CN or NO₂ and substituted on nitrogen by a straight hydrocarbon group of at least 6 carbon atoms which is unsubstituted or substituted by fluorine and the chain of which may comprise a group -O-, -S-, -CO-O-, -O-CO-, -NH-CO-, -CO-NH- or carbon to carbon double or triple bond, or R represents a phenyl group substituted in the 3- or 4-position by a group R as hereinbefore defined or by a corresponding group containing less than 6 carbon atoms; B is H, CH₃, F, Cl, Br, CN or NO₂; and D represents certain phenyl or naphthyl groups are photochromic compounds.式(I)化合物; 其中 A 代表某些未被或被 F、Cl、Br、CN 或 NO₂ 取代的吡啶鎓、喹啉鎓、异喹啉鎓或嘧啶鎓基团,在氮上被至少 6 个碳原子的直烃基取代,该直烃基团未被或被氟取代,其链可包括基团 -O-、-S-、-CO-O-、-O-CO-、-NH-CO-、-CO-NH- 或碳对碳双键或三键,或 R 代表苯基、-S-、-CO-O-、-O-CO-、-NH-CO-、-CO-NH- 或碳对碳双键或三键,或者 R 代表在 3 位或 4 位被前文定义的基团 R 或含少于 6 个碳原子的相应基团取代的苯基; B 是 H、CH₃、F、Cl、Br、CN 或 NO₂;以及 D 代表某些苯基或萘基的光致变色化合物。
-
Photochemical Regulation of the Electrical Properties of the Novel Planar Bilayer Lipid Membrane Incorporating Spiropyran Derivatives作者:Motomu Tanaka、Yoshiro YonezawaDOI:10.1021/jp953340t日期:1996.1.1We incorporated the alkyl chain substituted spiropyran derivatives, 1-methyl-3,3-dimethyl-6'-nitrospiro[indoline-2,2'-[2H][1]benzopyran] (SP1), 1-octadecyl-3,3-dimethyl-6'-nitrospiro[indoline-2,2'-[2H][1]benzopyran] (SP18), and 1-octadecyl-3,3-dimethyl-6'-nitro-8-[docosanoyloxymethyl]spiro[indoline-2,2'-[2H][1]benzopyran] (SP1822) into the planar bilayer lipid membranes (BLM) of soybean lecithin for the first time, and the photoresponse of the BLM was monitored. When a dc voltage was applied across the BLM, the transmembrane current changed under alternate irradiation with ultraviolet light and visible light. Such a photoelectric response of the BLM seems to be caused by the change in the membrane conductivity due to photoisomerization of spiropyran.
-
J-Aggregate Formation of Spiropyran Derivatives in LB and Vapor-Deposited Thin Films作者:Kouji Matsumoto、Takashi Shinohara、Yasuko Koshiba、Yasukiyo Ueda、Zhenguo JiDOI:10.1080/15421400500366753日期:2006.3J-aggregate formation in the films of spiropyran derivatives ( SP1822 and SP18) prepared with Langumuir-Blodegett ( LB) and vapor-deposition methods was investigated. In the films of SP1822, J-aggregate formation occurred with UV light illumination and thermal treatment. In the case of SP18 film, on the other hand, only photoisomerization occurred. When the PMC-form of SP18 was transferred onto a glass plate, J-like aggregate formation occurred after UV light illumination. By UV light illumination during evaporation process, SP1822 formed J-aggregate. Finally, SP1822 J-aggregate film oriented one-dimensionally was fabricated by using a PTFE layer as a substrate.
-
Temperature Effect on Photochromic Reaction in Langmuir−Blodgett Films of Amphiphilic Spiropyran and Their Morphological Changes作者:Hiroaki Tachibana、Yasushi Yamanaka、Mutsuyoshi MatsumotoDOI:10.1021/jp0104841日期:2001.10.1The effect of temperature on photochromic reaction of amphiphilic spiropyran, 1',3'-dihydro-3',3'-dimethyl-6-nitro-1'-octadecyl-8-(docosanoyloxyme thyl)spiro [2H-1-benzopyran-2,2'-(2H)-indole] (SP1822), was investigated in single-component LB films. The morphological changes accompanying the photochromic reaction of SP1822 in the LB films were measured by atomic force microscopy (AFM) and scanning near-field optical microscopy (SNOM). Many circular domains with a width of 10-20 mum and a height of 4-5 nm were observed in as-deposited LB films before irradiation. Surface structures on circular domain did not change accompanying isomerization of SP1822 to open-colored photomerocyanine (PMC) on irradiation with UV light at room temperature, but the SNOM image reveals that the isomerization to PMC occurs into the circular domains. After reaching the photostationary state of PMC, further addition of heat at 50 degreesC during UV irradiation induces J-aggregate of PMC. The AFM shows that many cone-shaped structures with average width of 0.6 mum and average height of 40 nm grow up from the circular domain. We have clarified that the heating of the sample at 50 degreesC during UV irradiation is necessary for the PMC to form J-aggregates.
-
Formation of Metal-free J-aggregates in Merocyanine/Spiropyran Mixed Langmuir-Blodgett Film作者:Misako Masui、Yasuko Koshiba、Yasukiyo Ueda、Zhenguo JiDOI:10.1080/15421400701545288日期:2007.8.20In order to fabricate metal-free J-aggregate, merocyanine(MD)/spiropyran(SP) mixed LB film was transferred on a glass plate from a pure aqueous solution. Upon UV-light illumination, SP molecules isomerized from SP-form to photomerocyanine (PMC)-form and PMC-form molecules formed J-aggregate with self-assembly. Subsequent vapor-treatment of dimethylamine (DMA) aqueous solution after UV-light illumination induced J-aggregate formation of MD molecules. Metal-free J-aggregate of MD dye was formed by the stepwise treatment of UV illumination and the DMA vapor treatment. The formation mechanism of J-aggregate was discussed.
表征谱图
-
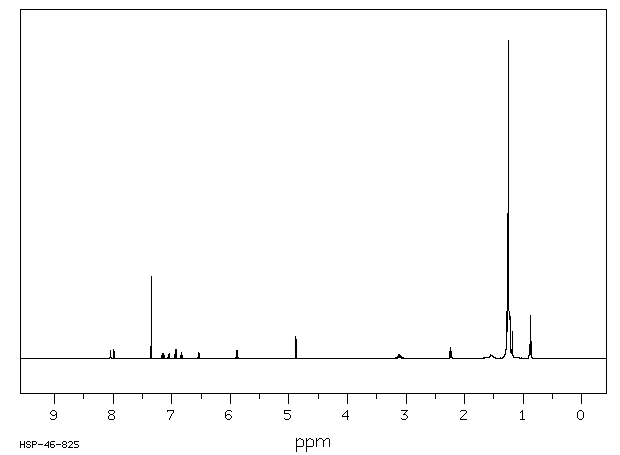
氢谱1HNMR
-
质谱MS
-
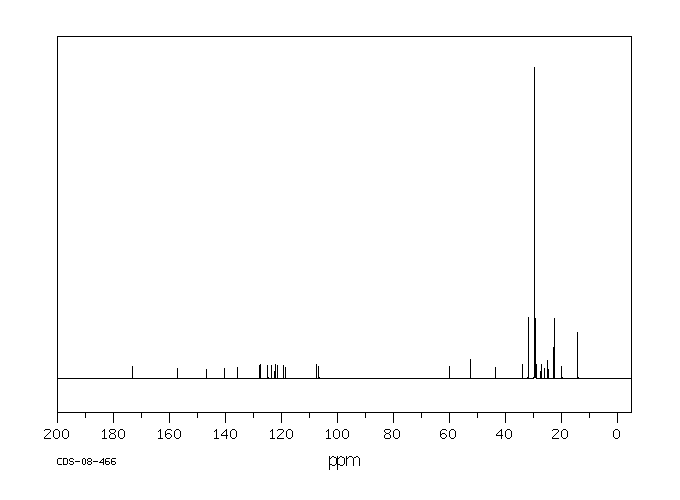
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-8-氟苯并二氢吡喃-4-胺
(2S)-6-氟-3,4-二氢-4-氧代-2H-1-苯并吡喃-2-甲酸
(2R)-6-氟-3,4-二氢-4-氧代-2H-1-苯并吡喃-2-甲酸
龙胆根素
龙胆SHAN酮
齿阿米素
黑色素-1
黄天精
麥角黃酮酸
鲍迪木醌
高邻苯二甲酸酐
高芒果苷
高氯酸罗丹明640
马佐卡林
香豆素-340
香豆素 339
食用色素红色105号
颜料红90:1铝色淀[CI45380:3]
颜料红172铝色淀[CI45430:1]
雏菊叶龙胆酮
降阿赛里奥; 1,3,6,7-四羟基氧杂蒽酮
阿米醇
阿米凯林
阿米凯林
阿扎那托
阿巴哌酮
阿尼地坦
阿尼地坦
阿匹氯铵
锌离子荧光探针-4
锆(2+)二[2-(2,4,5,7-四溴-3,6-二羟基氧杂蒽-9-基)-3,4,5,6-四氯苯甲酸酯]
铁力木呫吨酮-B
铁力木吨酮A
钠6'-羟基-5-[2-[4-(2-甲基-3-氧代-7H-咪唑并[1,2-d]吡嗪-6-基)苯氧基]乙基硫代氨基甲酰氨基]-3-氧代螺[2-苯并呋喃-1,9'-氧杂蒽]-3'-醇
钠4-{[6'-(二乙基氨基)-3'-羟基-3-氧代-3H-螺[2-苯并呋喃-1,9'-氧杂蒽]-2'-基]偶氮}-3-羟基-1-萘磺酸酯
钠2-(2,7-二氯-9H-氧杂蒽-9-基)苯甲酸酯
钙黄绿素乙酰甲酯
钙荧光探针Fluo-8,AM
钙荧光探针Fluo-4,AM
钙离子荧光探针
钙柑子
采木(HAEMATOXYLONCAMPECHIANUM)木质提取物
酸性媒介桃红3BM
邻苯三酚红
远志山酮III
转移核糖核酸(面包酵母)
赤藓红B异硫氰酸酯异构体II
赤藓红
诺大麻
西伯尔链接剂