4-羟苯基二甲基锍甲磺酸盐 | 32279-04-2
中文名称
4-羟苯基二甲基锍甲磺酸盐
中文别名
4-羟基二甲基硫苯甲基磺酸酯
英文名称
(p-hydroxyphenyl)dimethylsulfonium methyl sulfate
英文别名
(4-hydroxyphenyl)dimethylsulfonium methyl sulfate;4-(Dimethyl-lambda4-sulfanylidene)cyclohexa-2,5-dien-1-one;methyl hydrogen sulfate;4-(dimethyl-λ4-sulfanylidene)cyclohexa-2,5-dien-1-one;methyl hydrogen sulfate
CAS
32279-04-2
化学式
CH3O4S*C8H11OS
mdl
——
分子量
266.339
InChiKey
PKQZVASULXKBJV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:92 °C
-
稳定性/保质期:
如果遵照规格使用和储存,则不会分解,未有已知危险反应。
计算性质
-
辛醇/水分配系数(LogP):0.72
-
重原子数:16
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.33
-
拓扑面积:96
-
氢给体数:1
-
氢受体数:5
安全信息
-
海关编码:2930909090
SDS
4-羟苯基二甲基锍甲磺酸盐 修改号码:5
模块 1. 化学品
产品名称: 4-Hydroxyphenyldimethylsulfonium Methyl Sulfate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4-羟苯基二甲基锍甲磺酸盐
百分比: >98.0%(T)
CAS编码: 32279-04-2
俗名: Dimethyl-4-hydroxyphenylsulfonium Methyl Sulfate
4-羟苯基二甲基锍甲磺酸盐 修改号码:5
模块 3. 成分/组成信息
分子式: C9H14O5S2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体-粉末
4-羟苯基二甲基锍甲磺酸盐 修改号码:5
模块 9. 理化特性
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点:
92°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
4-羟苯基二甲基锍甲磺酸盐 修改号码:5
模块 14. 运输信息
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 4-Hydroxyphenyldimethylsulfonium Methyl Sulfate
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4-羟苯基二甲基锍甲磺酸盐
百分比: >98.0%(T)
CAS编码: 32279-04-2
俗名: Dimethyl-4-hydroxyphenylsulfonium Methyl Sulfate
4-羟苯基二甲基锍甲磺酸盐 修改号码:5
模块 3. 成分/组成信息
分子式: C9H14O5S2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体-粉末
4-羟苯基二甲基锍甲磺酸盐 修改号码:5
模块 9. 理化特性
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点:
92°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 硫氧化物
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
4-羟苯基二甲基锍甲磺酸盐 修改号码:5
模块 14. 运输信息
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:参考文献:名称:水溶液中的肽合成。四、[对(苄氧羰基氧基)苯基]二甲基硫酸锍(Z-ODSP)、[对(叔丁氧基羰氧基)苯基]二甲基硫酸锍(Boc-ODSP)和[对(9-芴基甲氧基羰基氧基)苯基]的制备及性质硫酸甲酯二甲基锍 (Fmoc-ODSP) 作为水溶性 N-酰化试剂摘要:为了增加水溶性活性酯法的应用,水溶性酰化试剂[对(苄氧基羰基氧基)苯基]二甲基硫酸锍、[对(叔丁氧基羰氧基)苯基]二甲基硫酸锍和[对- (9-芴基甲氧基羰基氧基)苯基]二甲基锍甲基硫酸盐由对羟基苯基二甲基锍甲基硫酸盐合成。这些化合物在水中具有高溶解度(超过 30%),并且被发现可作为优异的水溶性酰化试剂,用于在水溶液中合成 N-苄氧羰基、N-叔丁氧羰基、N-9-芴基甲氧羰基氨基酸衍生物。他们在水性介质和氨基酸中将二肽转化为 N-酰基二肽。此外,还发现它们是保护赖氨酸、鸟氨酸、DOI:10.1246/bcsj.62.3103
-
作为产物:描述:参考文献:名称:The Effect of m-Dichloro and m-Dibromo Groups on the Dissociation and Ultraviolet Spectra of p-Dimethylsulfoniophenols摘要:DOI:10.1021/ja01551a045
文献信息
-
Peptide Synthesis in Aqueous Solution. II. Synthesis and Biological Activity of a Molluscan Neuropeptide, FMRFamide (Phe–Met–Arg–Phe–NH<sub>2</sub>) Analogs for N-Terminal Moiety作者:Katsushige Kouge、Haruko Soma、Yukari Katakai、Hideo Okai、Mayumi Takemoto、Yojiro MuneokaDOI:10.1246/bcsj.60.4343日期:1987.12In order to elucidate the contribution of the N-terminal moiety (Phe1 or Met2) of FMRFamide to the activity, 16 kinds of FMRFamide analogs were synthesized and their structure-activity relations are discussed. From the results, it was found that hydrophobic or bulky group in N-terminal contributes to the contractile effect, while the precise length of the side chain of amino acid at 2-position is due
-
Peptide Synthesis in Aqueous Solution. I. Application of<i>p</i>-Dialkylsulfoniophenols as a Water-Soluble Coupling Reagent作者:Katsushige Kouge、Tatsuya Koizumi、Hideo Okai、Tetsuo KatoDOI:10.1246/bcsj.60.2409日期:1987.7found to be an excellent coupling reagent having a water-soluble property and a high reactivity; it worked as satisfactory as usual active esters in regard to the reactivity, product purity, and racemization. The marked advantage of the HODMSP·MeSO4− active ester method was the fact that bifunctional residues such as Arg, Lys, Cys, and Tyr could be selectively acylated when the pH of the reaction mixture
-
Peptide Synthesis in Aqueous Solution. III. Synthesis and Biological Activity of Cyclohexylamide Derivatives of Peptides Related to a Molluscan Neuropeptide, FMRFamide (Phe–Met–Arg–Phe–NH<sub>2</sub>)作者:Katsushige Kouge、Yukari Katakai、Haruko Soma、Miyuki Kondo、Hideo Okai、Mayumi Takemoto、Yojiro MuneokaDOI:10.1246/bcsj.60.4351日期:1987.12C-terminal Phe4–NH2 of FMRFamide (Phe–Met–Arg–Phe–NH2) to the biological activity, 30 kinds of cyclohexylamide derivatives of peptides related to FMRFamide were synthesized and their FMRFamide-like activity was measured. From the results, it was found that the peptide which was placed at C-terminal Phe4–NH2 by D-Ala–CHA showed only a relaxing activity. Furthermore, it was recognized that Met–Arg–Asp–diCHA
-
Fairly Marked Enantioselectivity for the Hydrolysis of Amino Acid Esters by Chemically Modified Enzymes<sup>1</sup>作者:Yoshihiro Yano、Kenji Shimada、Jiro Okai、Koichi Goto、Yoko Matsumoto、Ryuichi UeokaDOI:10.1021/jo0265075日期:2003.2.1The hydrolysis (deacylation) of enantiomeric substrates by the chemically modified enzymes decanoyl-alpha-chymotrypsin and decanoyl-trypsin was studied. Reaction activity for decanoyl-alpha-chymotrypsin was lower than that for the native enzyme, although intriguingly the enantioselectivity was markedly enhanced as compared with the native enzyme. In particular, the apparently complete enantioselective
-
ONIUM SALT AND COMPOSITION COMPRISING THE SAME申请人:ASAHI KASEI E-MATERIALS CORPORATION公开号:US20160229801A1公开(公告)日:2016-08-11The onium salt of the present invention contains predetermined compound A represented by the general formula (1). The composition of the present invention contains the onium salt of the present invention, and an onium salt containing predetermined compound B represented by the general formula (2). The onium salt and the composition of the present invention exert well-balanced excellent physical properties in terms of cold curing properties, storage stability, thermal shock resistance after curing, and moisture resistance.
表征谱图
-
氢谱1HNMR
-
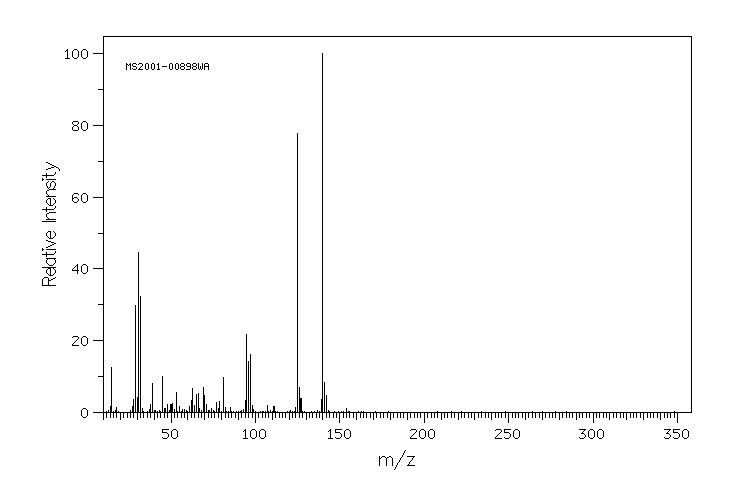
质谱MS
-
碳谱13CNMR
-
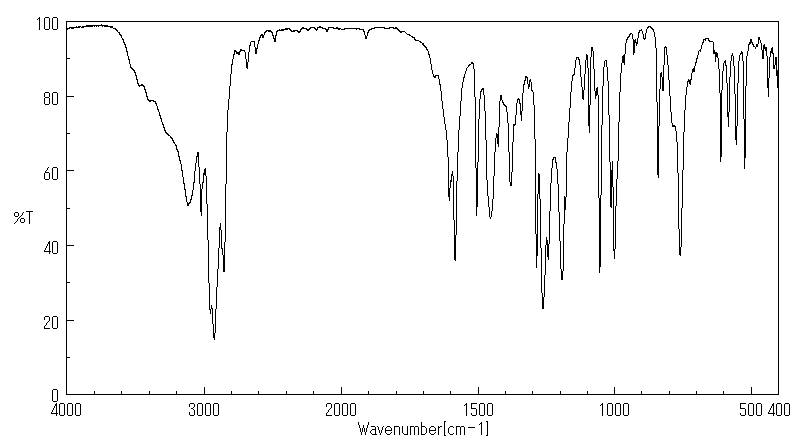
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚