4-methyl-5-(naphthalen-1-yl)thiazol-2-amine | 92149-93-4
中文名称
——
中文别名
——
英文名称
4-methyl-5-(naphthalen-1-yl)thiazol-2-amine
英文别名
4-Methyl-5-(1-naphthyl)-2-thiazolamine;4-methyl-5-naphthalen-1-yl-1,3-thiazol-2-amine
CAS
92149-93-4
化学式
C14H12N2S
mdl
——
分子量
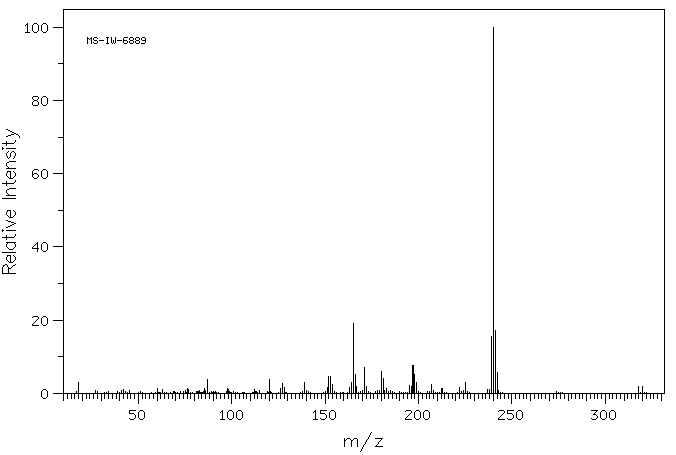
240.329
InChiKey
WANSLCSWLWQFFL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:163 °C
-
沸点:417.3±14.0 °C(Predicted)
-
密度:1.264±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:17
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.07
-
拓扑面积:67.2
-
氢给体数:1
-
氢受体数:3
反应信息
-
作为反应物:描述:4-methyl-5-(naphthalen-1-yl)thiazol-2-amine 、 丁炔二酸二甲酯 以 四氢呋喃 为溶剂, 反应 8.0h, 以76%的产率得到methyl 3-methyl-2-(naphthalen-1-yl)-7-oxo-7H-thiazolo[3,2-a]pyrimidine-5-carboxylate参考文献:名称:在温和条件下高度区域选择性合成7-oxo-7 H- [1,3,4]噻二唑[3,2 - a ]嘧啶-5-羧酸酯衍生物摘要:7-Oxo-7 H- [1,3,4]噻二唑[3,2- a]嘧啶-5-羧酸酯衍生物是生物学上和药理上有用的杂环。通过在超声波辐射的帮助下,2-氨基噻二唑与乙炔二羧酸二甲酯(DMAD)在THF中进行无催化剂的一锅反应,开发出了用于此类化合物的有效合成方法。该反应显示出适用于多种底物,并且相对于其“ 5-one”异构体,“ 7-one”产物具有较高的区域选择性。通过基于密度泛函理论(DFT)计算的理论模型分析,绘制出详细的反应机理。机理研究表明,有利的反应途径包括一系列氢键引导的迈克尔加成,协同质子转移/五元开环和分子内氰基杂-Diels-Alder反应。DOI:10.1016/j.tetlet.2019.04.005
-
作为产物:描述:1-(1'-naphthyl)-2-propyn-1-ol 在 吡啶 、 三氯化磷 作用下, 以 乙醇 为溶剂, 反应 2.0h, 生成 4-methyl-5-(naphthalen-1-yl)thiazol-2-amine参考文献:名称:炔属化合物的研究。二十二。闭环。(4)。噻唑和咪唑的新合成。摘要:研究发现,具有通式RCH(X)-C≡C-R'的化合物在反应中与硫脲作用生成噻唑衍生物。噻唑结构和取代基之间的关系阐明如下:(1) 当R=R'=Ph时,获得2-氨基-4-苄基-5-苯基噻唑(IId);(2) 当R=烷基,R'=H时,获得两种噻唑;(3) 当R=R'=烷基时,不生成噻唑衍生物。同样地,炔丙基溴和苯基炔丙基溴与氨荒酸盐反应,分别得到2-巯基-4-甲基噻唑(XX)和2-巯基-4-苄基噻唑(XXIII)。进一步地,从胍和炔丙基溴获得2-氨基-4-甲基咪唑(XXV)。DOI:10.1248/cpb.10.376
文献信息
-
One-pot three-component protocol for the synthesis of substituted 2-aminothiazoles作者:Shanshan Guo、Donghong Zhao、Yue Zhu、Yongping Yu、Wenteng Chen、Guolin ZhangDOI:10.1080/00397911.2017.1350275日期:2017.10.2ABSTRACT Substituted 2-aminothiazoles have been synthesized from α-nitro-epoxides, cyanamide, and sodium sulfide through a facile, three-component, and ecofriendly protocol with good to excellent yields. This reaction was achieved at room temperature without any additives. A possible mechanism has also been proposed. GRAPHICAL ABSTRACT
-
Efficient and facile strategy to substituted 2-aminothiazoles via ring opening of α-nitroepoxides作者:Yue Zhu、Qilin Wang、Haofan Luo、Guolin Zhang、Yongping YuDOI:10.1016/j.tet.2018.09.005日期:2018.12A novel and efficient reaction has been developed to synthesize a set of substituted 2-aminothiazoles from α-nitroepoxides and ammonium thiocyanate. This reaction could proceed smoothly at mild condition, to afford products for a wide range of substrates with good to excellent yields. A possible mechanism has also been proposed.
-
Okamiya, Nippon Kagaku Zasshi, 1959, vol. 80, p. 903,904作者:OkamiyaDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
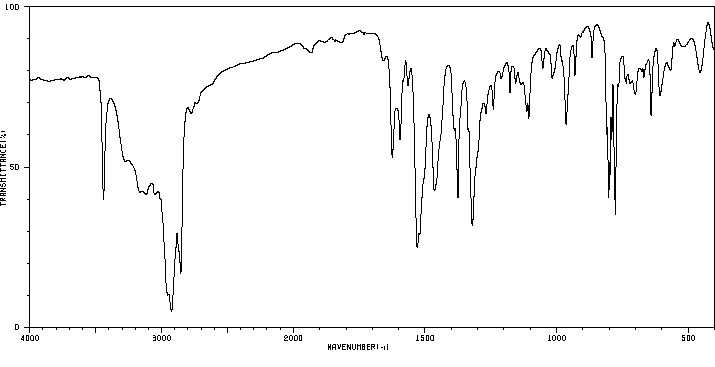
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮