(E)-ethyl 4-(phenyldiazenyl)benzoate | 37790-22-0
中文名称
——
中文别名
——
英文名称
(E)-ethyl 4-(phenyldiazenyl)benzoate
英文别名
ethyl (E)-4-(phenyldiazenyl)benzoate;ethyl 4-((E)-phenyldiazenyl)benzoate;(E)-4-(phenyldiazenyl) benzoate;4-(ethoxycarbonyl)azobenzene
CAS
37790-22-0
化学式
C15H14N2O2
mdl
——
分子量
254.288
InChiKey
ZABJMLAHGWTODF-WUKNDPDISA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):4.28
-
重原子数:19.0
-
可旋转键数:4.0
-
环数:2.0
-
sp3杂化的碳原子比例:0.13
-
拓扑面积:51.02
-
氢给体数:0.0
-
氢受体数:4.0
上下游信息
反应信息
-
作为反应物:描述:(E)-ethyl 4-(phenyldiazenyl)benzoate 在 N-碘代丁二酰亚胺 、 palladium diacetate 、 对甲苯磺酸 作用下, 以 硝基甲烷 为溶剂, 反应 20.0h, 以75%的产率得到(E)-ethyl 4-((2-iodophenyl)diazenyl)benzoate参考文献:名称:钯催化芳香偶氮化合物的区域选择性卤化摘要:对称或不对称芳族偶氮化合物的高度区域选择性卤化反应已在室温或50°C下进行了开发。在5mol%的二乙酸钯和0.5当量的存在下。在对甲苯磺酸的基础上,一系列对称的芳族偶氮化合物与N-溴代琥珀酰亚胺平稳地进行单溴化反应,从而以良好的产率和优异的邻位选择性> 99:1给出相应的不对称芳族偶氮化合物。该化学方法已经成功地扩展到不对称芳族偶氮化合物,其富电子芳基更倾向于单溴化。此外,用N代替N-溴琥珀酰亚胺反应中的-碘代琥珀酰亚胺允许合成具有> 99:1区域选择性的单碘代芳族偶氮化合物。DOI:10.1002/adsc.201200902
-
作为产物:描述:苯甲酸,4-(2-苯基肼基)-,乙基酯 在 双氧水 、 3C16H36N(1+)*FeMo6O18((OCH2)3CNH2)2(3-) 、 sodium hydrogensulfite 作用下, 以 乙醇 、 水 为溶剂, 反应 0.5h, 以84%的产率得到(E)-ethyl 4-(phenyldiazenyl)benzoate参考文献:名称:使用基于多氧钼酸盐的铁催化剂氧化肼和二芳胺的氧化脱氢摘要:我们报告了一种使用安德森型多氧钼酸铁 ( III ) 作为催化剂和过氧化氢作为氧化剂在乙醇水溶液中氧化脱氢肼和二芳基胺的有效方法。以中等至极好的收率获得了一系列偶氮化合物和四芳基肼。反应条件和底物范围补充或优于更成熟的方案。此外,该催化剂在水中表现出良好的稳定性和重复使用性。初步的机理研究表明,该反应涉及一个自由基过程。DOI:10.1039/d1cc02753k
文献信息
-
First use of p-tert-butylcalix[4]arene-tetra-O-acetate as a nanoreactor having tunable selectivity towards cross azo-compounds by trapping silver ions作者:Piyali Sarkar、Chhanda MukhopadhyayDOI:10.1039/c5gc01859e日期:——
p-tert -Butylcalix[4]arene-tetra-O -acetate was established for the first time as a member of the nanoreactor series, even without having any –OH group.p-tert -Butylcalix[4]arene-tetra-O -acetate首次被确定为纳米反应器系列的一员,即使没有任何-OH基团。 -
Direct Access to Acylated Azobenzenes and Amide Compounds by Reaction of Azoarenes with Benzylic Ethers as Acyl Equivalents作者:Shengying Wu、Limin Wang、Gang Hong、Alfred Aruma、Xiaoyan ZhuDOI:10.1055/s-0035-1561380日期:——pathway for the Pd-catalyzed regiospecific ortho-acylation of azoarenes using benzylic ethers as acyl equivalents has been achieved. In the absence of palladium catalyst, amide compounds were formed by the reaction of azoarenes with benzylic ethers under certain conditions. Various mono-acylazobenzene and amide compounds were obtained in good yields (35 examples). The mono-acylated products and amide摘要 本文描述了首次在一个反应方案中使用偶氮苯的N = N双键作为导向基团和自由基受体。已经实现了使用苄基醚作为酰基当量的Pd催化的偶氮芳烃区域特异性邻位酰化的有效途径。在不存在钯催化剂的情况下,通过在一定条件下偶氮芳烃与苄基醚反应形成酰胺化合物。以高收率获得了各种单酰基唑苯和酰胺化合物(35个实例)。单酰化产物和酰胺产物可以通过钯催化剂容易地控制。 本文描述了首次在一个反应方案中使用偶氮苯的N = N双键作为导向基团和自由基受体。已经实现了使用苄基醚作为酰基当量的Pd催化的偶氮芳烃区域特异性邻位酰化的有效途径。在不存在钯催化剂的情况下,通过在一定条件下偶氮芳烃与苄基醚反应形成酰胺化合物。以高收率获得了各种单酰基唑苯和酰胺化合物(35个实例)。单酰化产物和酰胺产物可以通过钯催化剂容易地控制。
-
Copper-Catalyzed Aerobic Oxidative Dehydrogenative Coupling of Anilines Leading to Aromatic Azo Compounds using Dioxygen as an Oxidant作者:Chun Zhang、Ning JiaoDOI:10.1002/anie.201001651日期:2010.8.16In the air tonight: A novel approach to symmetric and unsymmetric aromatic azo compounds from simple anilines catalyzed by inexpensive CuBr has been disclosed. Air (or dioxygen) was used as an oxidant under mild reaction conditions, with H2O as the byproduct, to make this transformation environmentally benign and very easy to handle.
-
Palladium-Catalyzed Oxidative Synthesis of Unsymmetrical Azophenols作者:Thi Hong Long Nguyen、Nicolas Gigant、Sandrine Delarue-Cochin、Delphine JosephDOI:10.1021/acs.joc.5b02614日期:2016.3.4reported. The developed methodology tolerates various functional groups and allows the synthesis of diverse unsymmetrical azophenols under mild conditions in good to excellent yields. A complementary procedure was also investigated by in situ generation of PIFA. This study represents the first general method for the synthesis of o-hydroxyazobenzenes starting from simple azoarenes.
-
Visible-Light-Mediated Hydroacylation of Azobenzenes with α-Keto Acids作者:Jingya Yang、Menghui Song、Hongyan Zhou、Ganggang Wang、Ben Ma、Yuanyuan Qi、Congde HuoDOI:10.1021/acs.orglett.0c03039日期:2020.11.6A visible-light-mediated protocol for the hydroacylation of azobenzenes with α-keto acids has been developed. In the absence of any catalyst or additive, decarboxylative hydroacylation proceeded smoothly under visible-light irradiation at room temperature. A wide range of azobenzenes and α-keto acids were well-tolerated and afforded hydroacylation products in high to excellent yields. Preliminary investigations
表征谱图
-
氢谱1HNMR
-
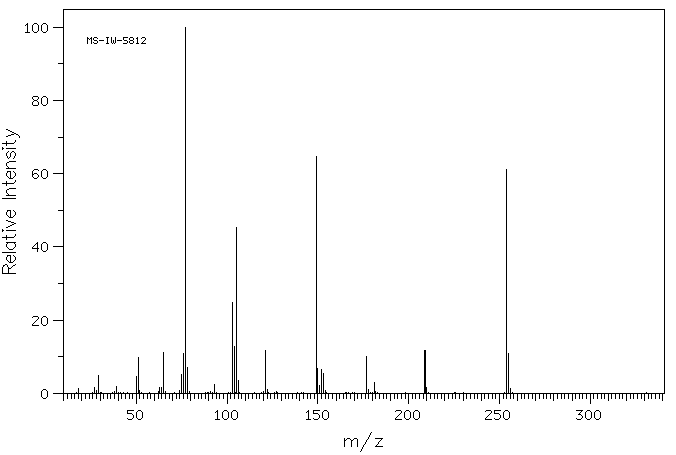
质谱MS
-
碳谱13CNMR
-
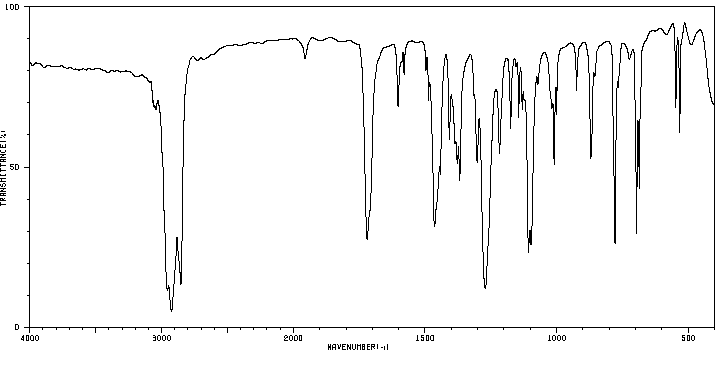
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黑洞猝灭剂-2,BHQ-2ACID
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S,24S)-
颜料橙61
阿利新黄GXS
阳离子红X-GTL
阳离子红5BL
阳离子橙RN
阳离子橙GLH
间甲基红
镨(3+)丙烯酰酸酯
镍酸酯(1-),[3-羟基-4-[(4-甲基-3-硫代苯基)偶氮]-2-萘羧酸根(3-)]-,氢
锂3-({4-[(4-羟基苯基)偶氮]-5-甲氧基-2-甲基苯基}偶氮)苯磺酸酯
钴,[二[m-[[1,2-二苯基-1,2-乙二酮1,2-二(肟酸根-kO)](2-)]]四氟二硼酸根(2-)-kN1,kN1',k2,kN2']-,(SP-4-1)-
钠5-氯-2-羟基-3-[(2-羟基-4-{[(4-甲基苯基)磺酰基]氧基}苯基)偶氮]苯磺酸酯
钠5-[[3-[[5-[[4-[[[4-[(4,5-二氢-3-甲基-5-氧代-1H-吡唑-4-基)偶氮]苯基]氨基]羰基]苯基]偶氮]-2,4-二羟基苯基]偶氮]-4-羟基苯基]偶氮]水杨酸盐
钠4-[(4-氨基苯基)偶氮]苯甲酸酯
钠4-[(4-{[4-(二乙基氨基)苯基]偶氮}苯基)偶氮]苯磺酸酯
钠4-[(4-{[2-羟基-5-(2-甲基-2-丙基)苯基]偶氮}苯基)偶氮]苯磺酸酯
钠4-({3-甲氧基-4-[(4-甲氧基苯基)偶氮]苯基}偶氮)苯磺酸酯
钠3-[4-(2-羟基-5-甲基-苯基)偶氮苯基]偶氮苯磺酸酯
钠3-({5-甲氧基-4-[(4-甲氧基苯基)偶氮]-2-甲基苯基}偶氮)苯磺酸酯
钠3-({4-[(4-羟基-2-甲基苯基)偶氮]-3-甲氧基苯基}偶氮)苯磺酸酯
金莲橙O
重氮基烯,苯基[4-(三氟甲基)苯基]-
重氮基烯,二[4-(1-甲基乙基)苯基]-,(Z)-
重氮基烯,二[4-(1-甲基乙基)苯基]-,(E)-
重氮基烯,[4-[(2-乙基己基)氧代]-2,5-二甲基苯基](4-硝基苯基)-
重氮基烯,1,2-二(4-丙氧基苯基)-,(1E)-
重氮基烯,(2-氯苯基)苯基-
酸性金黄G
酸性棕S-BL
酸性媒染棕
酸性媒介棕6
酸性媒介棕48
酸性媒介棕4
酸性媒介棕24
邻氨基偶氮甲苯
达布氨乙基甲硫基磺酸盐
赛甲氧星
茴香酸盐己基
茜素黄 R 钠盐
苯重氮化,2-甲氧基-5-甲基-4-[(4-甲基-2-硝基苯基)偶氮]-,氯化
苯酰胺,4-[4-(2,3-二氢-1,4-苯并二噁英-6-基)-5-(2-吡啶基)-1H-咪唑-2-基]-
苯酚,4-(1,1-二甲基乙基)-2-(苯偶氮基)-
苯酚,2-甲氧基-4-[(4-硝基苯基)偶氮]-
苯胺棕
苯胺,4-[(4-氯-2-硝基苯基)偶氮]-
苯磺酸,3,3-6-(4-吗啉基)-1,3,5-三嗪-2,4-二基二亚氨基2-(乙酰基氨基)-4,1-亚苯基偶氮二-,盐二钠
苯磺酸,2-[(4-氨基-2-羟基苯基)偶氮]-
苯甲酸,5-[[4-[(乙酰基氨基)磺酰]苯基]偶氮]-2-[[3-(三氟甲基)苯基]氨基]-(9CI)