1-methoxy-4-thiocyanato naphthalene | 66231-77-4
中文名称
——
中文别名
——
英文名称
1-methoxy-4-thiocyanato naphthalene
英文别名
1-methoxy-4-thiocyanatonaphthalene;4-methoxy-[1]naphthylsulfanyl cyanate;4-Methoxy-[1]naphthylthiocyanat;4-methoxy-1-naphthyl thiocyanate;1-Methoxy-4-thiocyanato-naphthalin;1-Methoxy-4-thiocyanatonaphthalin;(4-methoxynaphthalen-1-yl) thiocyanate
CAS
66231-77-4
化学式
C12H9NOS
mdl
——
分子量
215.276
InChiKey
AQBMFRXFXLTEOL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:15
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.08
-
拓扑面积:58.3
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为反应物:描述:1-methoxy-4-thiocyanato naphthalene 在 sodium trisulfide 、 乙醇 作用下, 生成 1,2-bis(4-methoxynaphthalen-1-yl)disulfane参考文献:名称:Takiura et al., Yakugaku Zasshi/Journal of the Pharmaceutical Society of Japan, 1954, vol. 74, p. 843,845摘要:DOI:
-
作为产物:描述:参考文献:名称:Phenylsulfonyl nitromethanes as aldose reductase inhibitors摘要:这项发明涉及用于治疗糖尿病和半乳糖血症的某些并发症的新型药物组合物,其中包含硝基甲烷衍生物(或其无毒盐)作为活性成分。硝基甲烷衍生物是醛糖还原酶的抑制剂。许多抑制剂是新颖的,并且作为该发明的进一步特征,提供了它们的制造和使用过程。公开号:US05153227A1
文献信息
-
The Aromatic Thiocyanation of 1-Alkoxynaphthalene by the Copper(II) Thiocyanate Method作者:Kiyoko FujikiDOI:10.1246/bcsj.50.3065日期:1977.11A facile thiocyanation of 1-alkoxynaphthalene using copper(II) thiocyanate afforded 4-thiocyanato compound. The reaction proceeded at a moderate temperature (80–90 °C) even for 1-alkoxynaphthalene with higher alkyl groups (C6, C8, and C12).
-
Nitrogen Dioxide Catalyzed Oxidative Thiocyanation of Arenes with Ambient Air as the Terminal Oxidant作者:Yun-Lai Ren、Wenhui Wang、Bo Zhao、Xinzhe Tian、Shuang Zhao、Jianji Wang、Fuwei LiDOI:10.1002/cctc.201600785日期:2016.11.8NO2 is an effective catalyst for the oxidative thiocyanation of arenes. This unique catalyst is inexpensive and separated easily from the final products because of its low boiling point. The mild reaction conditions allow a series of arenes and thiophenes to be thiocyanated smoothly in moderate to high yields. A preliminary mechanistic investigation suggests that the present reaction may proceed through
-
Ammonium metavanadate/thiocyanate-triggered electrophilic thiocyanation of aromatic and heteroaromatic compounds in aqueous bisulfate and acetonitrile media作者:N. Venkatesham、K. Rajendar Reddy、K. C. Rajanna、P. VeerasomaiahDOI:10.1080/17415993.2014.940350日期:2014.11.2The ammonium metavanadate/thiocyanate system is used as an efficient reagent for regioselective thiocyanation of aromatic and hetero aromatic compounds under conventional and nonconventional conditions such as ultrasonically assisted and microwave-assisted reactions. The reactions proceeded smoothly and afforded good yields of products with high regioselectivity. Longer reaction times (about 8 h) observed
-
An Efficient Method for Thiocyanation of Aromatic and Heteroaromatic Compounds using Cyanuric Chloride and Ammonium Thiocyanate under Conventional and Nonconventional Conditions作者:K. Rajanna、P. Venkanna、M. Kumar、M. Venkateswarlu、M. AliDOI:10.1055/s-0035-1560503日期:——Highly efficient thiocyanation of aromatic and heteroaromatic compounds has been accomplished by using cyanuric chloride (NCCl)3/NH4SCN in dichloromethane under conventional and ultrasonic-assisted conditions. Sonicated reactions reached completion with reduced reaction times. The protocol involves a simple workup.
-
Poly[4-diacetoxyiodo] Styrene–Promoted Thiocyanation of Aromatic Ethers, Anilines, and Indoles作者:Liqiang Wu、Shujun Chao、Xiao Wang、Fulin YanDOI:10.1080/10426507.2010.496748日期:2011.1.31Abstract Thiocyanation of aromatic ethers, anilines, and indoles have been achieved using ammonium thiocyanate in the presence of poly[4-diacetoxyiodo] styrene (PDAIS) in CH3CN at room temperature. GRAPHICAL ABSTRACT
表征谱图
-
氢谱1HNMR
-
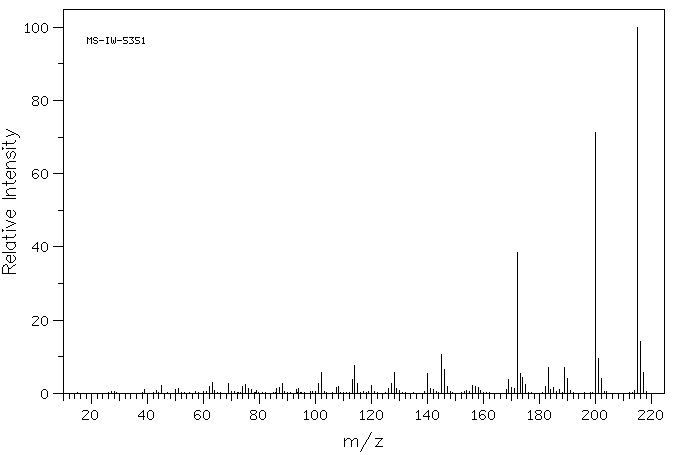
质谱MS
-
碳谱13CNMR
-
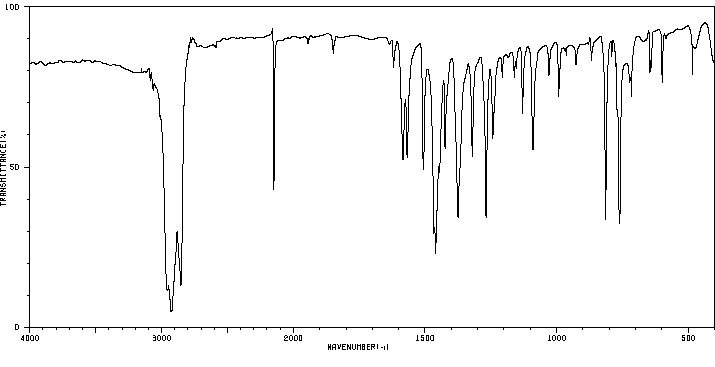
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮