2-(piperidin-1-yl)phenazine | 21942-84-7
中文名称
——
中文别名
——
英文名称
2-(piperidin-1-yl)phenazine
英文别名
2,N-Piperidylphenazin;2-Piperidino-phenazin;2-Piperidinophenazine;2-piperidin-1-ylphenazine
CAS
21942-84-7
化学式
C17H17N3
mdl
——
分子量
263.342
InChiKey
QGKHLZQPUCAWSY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:20
-
可旋转键数:1
-
环数:4.0
-
sp3杂化的碳原子比例:0.29
-
拓扑面积:29
-
氢给体数:0
-
氢受体数:3
反应信息
-
作为产物:参考文献:名称:使用可重复使用的钴催化剂通过苯胺和邻苯二胺的氧化环化直接获得功能性吩嗪摘要:尽管氨基吩嗪具有广泛的应用,但由于原料的可用性有限以及控制合成选择性方面的挑战,仍然缺乏一种能够直接和多样化地获取此类化合物的有效方法。在这里,通过使用单电子氧化 (SEO) 诱导串联双 C-H 胺化的策略,我们报告了一种温和的方法,通过容易获得的苯胺和邻苯二胺的氧化环化来解决上述问题,该方法使用广泛的底物进行范围和良好的官能团耐受性,直接使用伯胺作为C-H胺化剂,采用可重复使用的氮掺杂碳负载钴纳米催化剂(Co-Nx / NC-800)和天然丰富的分子O 2,并表现出出色的步进和原子效率。发达的化学为构建各种难以用常规方法合成的有前途的氨基吩嗪提供了一个实用的平台,并为在可持续多相贱金属催化下进一步开发 SEO 诱导的有价值的转化铺平了道路。DOI:10.1039/d2gc03878a
文献信息
-
Electrochemical Oxidative (4 + 2) Cyclization of Anilines and o-Phenylenediamines for the Synthesis of Phenazines作者:Peng-Fei Huang、Jia-Le Fu、Ying Peng、Ke-Wen Tang、Yu LiuDOI:10.1021/acs.orglett.4c00851日期:2024.5.10constituents of nitrogen-containing heterocycles, widely exist in functional compounds. Herein, we report an anodic oxidative (4 + 2) cyclization between anilines and o-phenylenediamines for the uniform construction of phenazines in a simple undivided cell. Dual C–H amination followed by oxidation represents an outstanding step and atom efficiency. A sequence of phenazines is produced with excellent functional
表征谱图
-
氢谱1HNMR
-
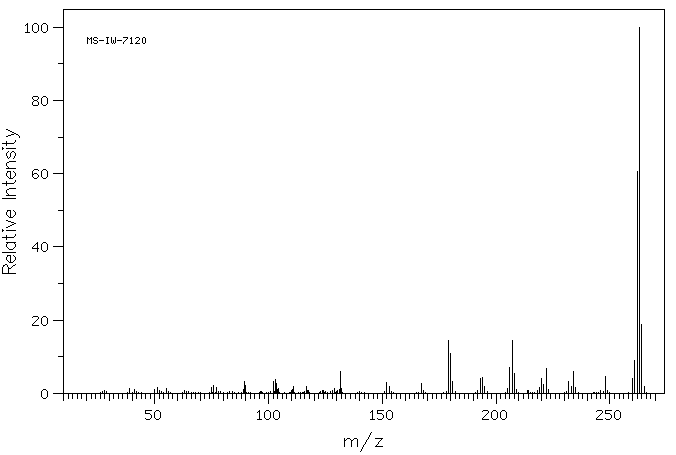
质谱MS
-
碳谱13CNMR
-
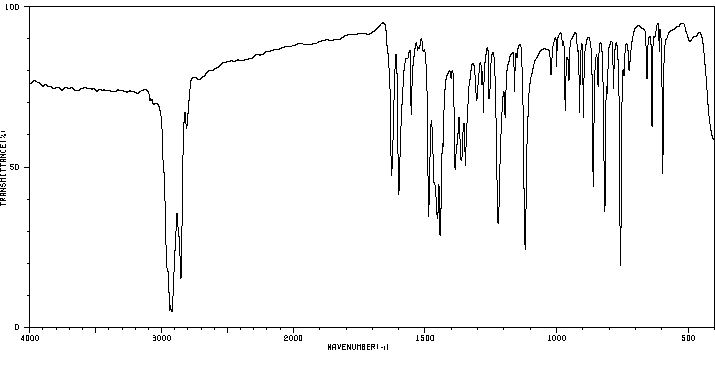
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(12羟基吲[2,1-b〕喹唑啉-6(12H)-酮)
黑暗猝灭剂BHQ-3,BHQ-3NHS
鸭嘴花酚碱
鸭嘴花碱酮;(S)-2,3-二氢-3,7-二羟基吡咯并[2,1-b]喹唑啉-9(1H)-酮
鸭嘴花碱酮
鸭嘴花碱盐酸盐
鲁米诺单钠盐
鲁米诺
骆驼蓬碱
颜料蓝64
颜料蓝60
顺式-卤夫酮
顺式-(喹喔啉-2-基)丙烯腈1,4-二氧化物
非奈利酮
青黛酮
雷替曲塞杂质1
阿法替尼杂质J
阿法替尼杂质I
阿法替尼杂质28
阿法替尼杂质18
阿法替尼杂质13
阿法替尼杂质
阿法替尼中间体
阿法替尼
阿法替尼
阿朴藏红
阿巴康唑
阿夫唑嗪杂质A
阿夫唑嗪杂质
阿夫唑嗪EP杂质C
阿夫唑嗪
阿喹司特
阿呋唑嗪杂质
阿呋唑嗪杂质
铜迈星
铁诱导细胞死亡激活剂
钠四丙基硼酸酯
酸性蓝98
酸性红101
酮色林醇
酞联氮基[2,3-b]酞嗪-5,14-二酮,7,12-二氢-
酞嗪-5-羧酸
酞嗪-2-氧化物
酚藏花红
酚嗪
酒石酸溴莫尼定
邻苯二甲酰肼
还原黄6GD
还原蓝6
达尼喹酮