5-氯-3-[(5-苯基-1,3,4-噁二唑-2-基)氨基]-2H-吲哚-2-酮 | 16713-15-8
中文名称
5-氯-3-[(5-苯基-1,3,4-噁二唑-2-基)氨基]-2H-吲哚-2-酮
中文别名
盐酸二乙基对苯二胺;显影剂TSS;对氨基二乙苯胺盐酸盐;盐酸对氨基二乙苯胺
英文名称
N,N-Diethyl-1,4-phenylenediamine Dihydrochloride
英文别名
4-N,4-N-diethylbenzene-1,4-diamine;dihydrochloride
CAS
16713-15-8;84609-46-1
化学式
C10H18Cl2N2
mdl
MFCD00060227
分子量
237.17
InChiKey
NHPCFKHZILUMDE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.97
-
重原子数:14
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:29.3
-
氢给体数:3
-
氢受体数:2
安全信息
-
海关编码:2921590090
SDS
反应信息
-
作为产物:描述:N,N-二乙基对苯二胺 、 盐酸 以 乙醚 为溶剂, 以to give N,N-diethyl-p-phenylenediamine dihydrochloride as a light brown solid (57.76 g, 100%)的产率得到5-氯-3-[(5-苯基-1,3,4-噁二唑-2-基)氨基]-2H-吲哚-2-酮参考文献:名称:METHODS OF CHEMICAL SYNTHESIS AND PURIFICATION OF DIAMINOPHENOTHIAZINIUM COMPOUNDS INCLUDING METHYLTHIONINIUM CHLORIDE (MTC)摘要:本发明涉及化学合成和纯化领域,更具体地涉及合成和纯化某些3,7-二氨基苯并噻嗪-5-ium化合物(以下简称“二氨基苯并噻嗪化合物”),包括氯化甲基亚蒙(MTC)(也称亚甲蓝)。在一种实施例中,该方法按顺序包括以下步骤:亚硝基化(NOS);亚硝基还原(NR);硫代磺酸形成(TSAF);氧化偶联(OC);Cr(VI)还原(CR);离子和中间体的分离和纯化(IAPOZI);环闭合(RC);氯化物盐形成(CSF);其中之一:硫化物处理(ST);二甲基二硫代氨基甲酸盐处理(DT);碳酸盐处理(CT);乙二胺四乙酸处理(EDTAT);有机萃取(OE);和重结晶(RX)。本发明还涉及所得到的(高纯度)化合物、包含它们的组合物(例如,片剂、胶囊)以及它们在灭活病原体、医疗治疗和诊断等方面的使用方法,例如用于tau病理、阿尔茨海默病(AD)、皮肤癌、黑色素瘤、病毒性疾病、细菌性疾病或原虫性疾病。公开号:US20110021510A1
文献信息
-
Methods of chemical synthesis and purification of diaminophenothiazinium compounds including methylthioninium chloride (MTC)申请人:Wischik M. Claude公开号:US20060287523A1公开(公告)日:2006-12-21This invention pertains generally to the field of chemical synthesis and purification, and more specifically to methods of synthesizing and purifying certain 3,7-diaminophenothiazin-5-ium compounds (referred to herein as “diaminophenothiazinium compounds”) including Methythioninium Chloride (MTC) (also known as Methylene Blue). In one embodiment, the method comprises the steps of, in order: nitrosylation (NOS); nitrosyl reduction (NR); thiosulfonic acid formation (TSAF); oxidative coupling (OC); Cr(VI) reduction (CR); isolation and purification of zwitterionic intermediate (IAPOZI); ring closure (RC); chloride salt formation (CSF); one of: sulphide treatment (ST); dimethyldithiocarbamate treatment (DT); carbonate treatment (CT); ethylenediaminetetraacetic acid treatment (EDTAT); organic extraction (OE); and recrystallisation (RX). The present invention also pertains to the resulting (high purity) compounds, compositions comprising them (e.g., tablets, capsules), and their use in methods of inactivating pathogens, and methods of medical treatment and diagnosis, etc., for example, for tauopathies, Alzheimer's disease (AD), skin cancer, melanoma, viral diseases, bacterial diseases, or protozoal diseases.本发明通常涉及化学合成和纯化领域,更具体地涉及合成和纯化某些3,7-二氨基苯并噻嗪-5-ium化合物(以下简称“二氨基苯并噻嗪化合物”),包括甲基硫脲氯化物(MTC)(也称亚甲蓝)。在一种实施例中,该方法按顺序包括以下步骤:亚硝酰化(NOS);亚硝基还原(NR);硫代磺酸形成(TSAF);氧化偶合(OC);Cr(VI)还原(CR);离子对中间体的分离和纯化(IAPOZI);环闭合(RC);氯化物盐形成(CSF);其中之一:硫化物处理(ST);二甲基二硫脲酸盐处理(DT);碳酸盐处理(CT);乙二胺四乙酸处理(EDTAT);有机萃取(OE);和重结晶(RX)。本发明还涉及所得到的(高纯度)化合物、包含它们的组合物(例如片剂、胶囊)以及它们在灭活病原体、医疗治疗和诊断等方法中的使用,例如用于tau病理、阿尔茨海默病(AD)、皮肤癌、黑色素瘤、病毒性疾病、细菌性疾病或原虫性疾病。
-
MEDICAL METHODS UTILISING HIGH PURITY DIAMINOPHENOTHIAZINIUM COMPOUNDS申请人:WisTa Laboratories Ltd.公开号:US20180078562A1公开(公告)日:2018-03-22This disclosure pertains generally to the field of chemical synthesis and purification, and more specifically to methods of synthesizing and purifying certain 3,7 diamino-phenothiazin-5-ium compounds (referred to herein as “diaminophenothiaziniumcompounds”) including Methythioninium Chloride (MTC) (also known as Methylene Blue). In one embodiment, the method comprises the steps of, in order: nitrosylation (NOS); nitrosyl reduction (NR); thiosulfonic acid formation (TSAF); oxidative coupling (OC); Cr(VI) reduction (CR); isolation and purification of zwitterionic intermediate (IAPOZI); ring closure (RC); chloride salt-formation (CSF); one of: sulphide treatment (ST); dimethyldithiocarbamate treatment (DT); carbonate treatment (CT); ethylenediaminetetraacetic acid treatment (EDTAT); organic extraction (OE); and recrystallisation (RX). Also disclosed resulting (high purity) compounds, compositions comprising them (e.g., tablets, capsules), and their use in methods of inactivating pathogens, and methods of medical treatment and diagnosis, etc., for example, for tauopathies, Alzheimer's disease (AD), skin cancer, melanoma, viral diseases, bacterial diseases, or protozoal diseases.本公开涉及化学合成和纯化领域,更具体地涉及合成和纯化某些3,7-二氨基苯并噻嗪-5-ium化合物(以下简称“二氨基苯并噻嗪化合物”),包括氯化甲亚硫胺(MTC)(也称亚甲蓝)。在一种实施例中,该方法依次包括以下步骤:硝基化(NOS);硝酰还原(NR);硫代磺酸形成(TSAF);氧化偶联(OC);Cr(VI)还原(CR);分离和纯化离子性中间体(IAPOZI);环闭合(RC);氯化物盐形成(CSF);其中之一:硫化物处理(ST);二甲基二硫代氨基甲酸盐处理(DT);碳酸盐处理(CT);乙二胺四乙酸盐处理(EDTAT);有机萃取(OE);和重结晶(RX)。还公开了得到的(高纯度)化合物,包括它们的组合物(例如,片剂,胶囊)以及它们在灭活病原体的方法、医疗治疗和诊断方法等方面的应用,例如用于tau病理学、阿尔茨海默病(AD)、皮肤癌、黑色素瘤、病毒性疾病、细菌性疾病或原虫病等。
-
HIGH PURITY DIAMINOPHENOTHIAZINIUM COMPOUNDS INCLUDING METHYLTHIONINIUM CHLORIDE (MTC)申请人:WisTa Laboratories Ltd.公开号:US20160129009A1公开(公告)日:2016-05-12This invention pertains generally to the field of chemical synthesis and purification, and more specifically to methods of synthesizing and purifying certain 3,7 diamino-phenothiazin-5-ium compounds (referred to herein as “diaminophenothiaziniumcompounds”) including Methythioninium Chloride (MTC) (also known as Methylene Blue). In one embodiment, the method comprises the steps of, in order: nitrosylation (NOS); nitrosyl reduction (NR); thiosulfonic acid formation (TSAF); oxidative coupling (OC); Cr(VI) reduction (CR); isolation and purification of zwitterionic intermediate (IAPOZI); ring closure (RC); chloride salt-formation (CSF); one of: sulphide treatment (ST); dimethyldithiocarbamate treatment (DT); carbonate treatment (CT); ethylenediaminetetraacetic acid treatment (EDTAT); organic extraction (OE); and recrystallisation (RX). The present invention also pertains to the resulting (high purity) compounds, compositions comprising them (e.g., tablets, capsules), and their use in methods of inactivating pathogens, and methods of medical treatment and diagnosis, etc., for example, for tauopathies, Alzheimer's disease (AD), skin cancer, melanoma, viral diseases, bacterial diseases, or protozoal diseases.本发明涉及化学合成和纯化领域,更具体地涉及合成和纯化某些3,7-二氨基苯并噻嗪-5-ium化合物(以下简称“二氨基苯并噻嗪化合物”),包括氯化甲亚硫胺(MTC)(也称为亚甲蓝)。在一种实施例中,该方法依次包括以下步骤:亚硝基化(NOS);亚硝基还原(NR);硫代磺酸形成(TSAF);氧化偶联(OC);Cr(VI)还原(CR);离子对中间体的分离和纯化(IAPOZI);环闭合(RC);氯化物盐形成(CSF);其中之一:硫化物处理(ST);二甲基二硫代氨基甲酸盐处理(DT);碳酸盐处理(CT);乙二胺四乙酸处理(EDTAT);有机萃取(OE);和再结晶(RX)。本发明还涉及所得到的(高纯度)化合物,包括它们的组合物(例如,片剂、胶囊),以及它们在灭活病原体、医疗治疗和诊断等方面的应用,例如用于tau病理、阿尔茨海默病(AD)、皮肤癌、黑色素瘤、病毒疾病、细菌疾病或原虫疾病。
-
Methods of Chemical Synthesis and Purification of Diaminophenothiazinium Compounds Including Methylthioninium Chloride (Mtc)申请人:Storey John Mervyn David公开号:US20080207603A1公开(公告)日:2008-08-28This invention pertains generally to the field of chemical synthesis and purification, and more specifically to methods of synthesizing and purifying certain 3,7 diamino-phenothiazin-5-ium compounds (referred to herein as “diaminophenothiazinium compounds”) including Methylthioninium Chloride (MTC) (also known as Methylene Blue). In one embodiment, the method comprises the steps of, in order: nitrosylation (NOS); nitrosyl reduction (NR); thiosulfonic acid formation (TSAF); oxidative coupling (OC); Cr(VI) reduction (CR); isolation and purification of zwitterionic intermediate (IAPOZI); ring closure (RC); chloride salt formation (CSF); one of: sulphide treatment (ST); dimethyldithiocarbamate treatment (DT); carbonate treatment (CT); ethylenediaminetetraacetic acid treatment (EDTAT); organic extraction (OE); and recrystallisation (RX). The present invention also pertains to the resulting (high purity) compounds, compositions comprising them (e.g., tablets, capsules), and their use in methods of inactivating pathogens, and methods of medical treatment and diagnosis, etc., for example, for tauopathies, Alzheimer's disease (AD), skin cancer, melanoma, viral diseases, bacterial diseases, or protozoal diseases. Wherein: each of R 1 and R 9 is independently selected from: —H; C 1-4 alkenyl; and halogenated C 1-4 alkyl; each of R 3NA and R 3NB is independently selected from: C 1-4 alkyl; C 2-4 alkenyl; and halogenated C 4-1 alkyl; each of R 7NA and R 7NB is independently selected from: C 1-4 alkyl; C 2-4 alkenyl; and halogenated C 1-4 alkyl; and X is one or more anionic counter ions to achieve electrical neutrality.本发明涉及化学合成和纯化领域,更具体地涉及合成和纯化某些3,7-二氨基苯并噻吩-5-ium化合物(以下简称“二氨基苯并噻吩化合物”),包括氯甲亚蓝(MTC)(也称为亚甲蓝)的方法。在一种实施例中,该方法依次包括以下步骤:亚硝基化(NOS);亚硝基还原(NR);硫代磺酸酯形成(TSAF);氧化偶合(OC);Cr(VI)还原(CR);离子对中间体的分离和纯化(IAPOZI);环闭合(RC);氯化物盐形成(CSF);其中之一:硫化物处理(ST);二甲基二硫脲处理(DT);碳酸盐处理(CT);乙二胺四乙酸处理(EDTAT);有机萃取(OE);和再结晶(RX)。本发明还涉及所得到的(高纯度)化合物,包括它们的组合物(例如,片剂,胶囊),以及它们在灭活病原体,医疗治疗和诊断等方法中的使用,例如,用于tau病理,阿尔茨海默病(AD),皮肤癌,黑色素瘤,病毒性疾病,细菌性疾病或原虫性疾病。其中:R1和R9各自独立地选自:—H;C1-4烯基;和卤代C1-4烷基;R3NA和R3NB各自独立地选自:C1-4烷基;C2-4烯基;和卤代C4-1烷基;R7NA和R7NB各自独立地选自:C1-4烷基;C2-4烯基;和卤代C1-4烷基;X是一个或多个阴离子对以达到电中性。
-
Methods of treatment of a tauopathy condition comprising the use of thioninium compounds申请人:Wista Laboratories Ltd.公开号:US07737138B2公开(公告)日:2010-06-15This invention pertains generally to the field of chemical synthesis and purification, and more specifically to methods of synthesizing and purifying certain 3,7-diaminophenothiazin-5-ium compounds (referred to herein as “diaminophenothiazinium compounds”) including Methythioninium Chloride (MTC) (also known as Methylene Blue). In one embodiment, the method comprises the steps of, in order: nitrosylation (NOS); nitrosyl reduction (NR); thiosulfonic acid formation (TSAF); oxidative coupling (OC); Cr(VI) reduction (CR); isolation and purification of zwitterionic intermediate (IAPOZI); ring closure (RC); chloride salt formation (CSF); one of: sulphide treatment (ST); dimethyldithiocarbamate treatment (DT); carbonate treatment (CT); ethylenediaminetetraacetic acid treatment (EDTAT); organic extraction (OE); and recrystallisation (RX). The present invention also pertains to the resulting (high purity) compounds, compositions comprising them (e.g., tablets, capsules), and their use in methods of inactivating pathogens, and methods of medical treatment and diagnosis, etc., for example, for tauopathies, Alzheimer's disease (AD), skin cancer, melanoma, viral diseases, bacterial diseases, or protozoal diseases.本发明涉及化学合成和纯化领域,更具体地涉及合成和纯化某些3,7-二氨基苯并噻嗪-5-ium化合物(以下简称“二氨基苯并噻嗪化合物”),包括氯化甲基硫菌青素(MTC)(也称为亚甲蓝)。在一种实施例中,该方法依次包括以下步骤:亚硝基化(NOS);亚硝基还原(NR);硫代磺酸形成(TSAF);氧化偶联(OC);Cr(VI)还原(CR);离子中间体的分离和纯化(IAPOZI);环闭合(RC);氯化物盐形成(CSF);以下其中之一:硫化物处理(ST);二甲基二硫代氨基甲酸盐处理(DT);碳酸盐处理(CT);乙二胺四乙酸处理(EDTAT);有机萃取(OE);以及重结晶(RX)。本发明还涉及所得到的(高纯度)化合物、包含它们的组合物(例如,片剂、胶囊)以及它们在灭活病原体、医疗治疗和诊断等方法中的使用,例如用于tau病理、阿尔茨海默病(AD)、皮肤癌、黑色素瘤、病毒性疾病、细菌性疾病或原虫性疾病。
表征谱图
-
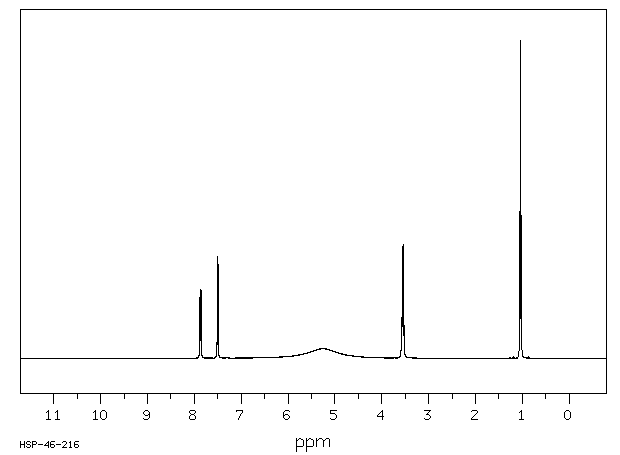
氢谱1HNMR
-
质谱MS
-
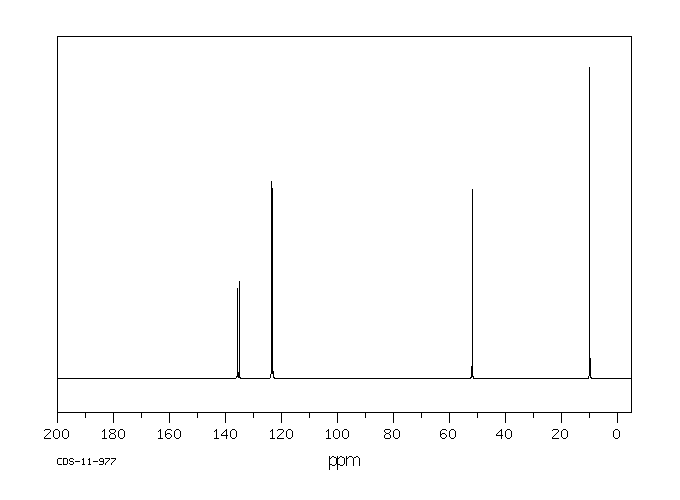
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷