5-甲基二硫杂环戊烯-3-硫酮 | 3354-40-3
中文名称
5-甲基二硫杂环戊烯-3-硫酮
中文别名
——
英文名称
5-methyl-[1,2]dithiole-3-thione
英文别名
5-methyl-3H-1,2-dithiole-3-thione;3H-1,2-Dithiole-3-thione, 5-methyl-;5-methyldithiole-3-thione
CAS
3354-40-3
化学式
C4H4S3
mdl
——
分子量
148.274
InChiKey
LEPDMIYUICCABX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:82.7
-
氢给体数:0
-
氢受体数:3
安全信息
-
海关编码:2930909090
SDS
反应信息
-
作为反应物:描述:5-甲基二硫杂环戊烯-3-硫酮 生成 5-[(E)-2-(4-tert-butylphenyl)ethenyl]dithiole-3-thione参考文献:名称:YAMAMOTO, ITARU;MATSUBARA, AKIRA;TOMIYA, KANDZI;MIDZUNO, OSAMU摘要:DOI:
-
作为产物:描述:参考文献:名称:Synthesis of 3H-1,2-dithiole-3-thiones by a novel oxidative cyclization摘要:Reaction of 3-oxo dithioic acids with a combination of hexamethyldisilathiane and N-chlorosuccinimide in the presence of a catalytic amount of imidazole brings about oxidative ring closure to 3H-1,2-dithiole-3-thiones. Yields vary from poor to good.DOI:10.1016/s0040-4039(00)79295-1
文献信息
-
Thionation with the Reagent Combination of Phosphorus Pentasulfide and Hexamethyldisiloxane作者:Thomas J. CurpheyDOI:10.1021/jo0256742日期:2002.9.1The combination of P4S10 and hexamethyldisiloxane efficiently converts esters, lactones, amides, lactams, and ketones to their corresponding thiono derivatives. In the presence of elemental sulfur, 3-oxoesters are converted to dithiolethiones by this reagent. Yields are comparable to or superior to those obtained with Lawesson's reagent. The method has the advantage that reagent-derived byproducts
-
Anethole dithiolethione and other dithiolethiones for the treatment of conditions associated with dysfunction of monoamine neruotransmission申请人:Drukarch Benjamin公开号:US20060194971A1公开(公告)日:2006-08-31The present disclosure relates to dithiolethione derivatives as monoamino oxidase inhibitors, in particular MAO-B inhibitors, to methods for the preparation of these compounds and to novel intermediates useful for the synthesis of said dithiolethiones derivatives. The present disclosure also relates to the use of a compound disclosed herein for the manufacture of a medicament giving a beneficial effect. In embodiments of the present disclosure specific compounds disclosed herein are used for the manufacture of a medicament useful in the treatment, amelioration or prevention of conditions associated with dysfunction of monoamine neurotransmission. The compounds have the general formula (1) wherein the symbols have the meanings given in the specification.
-
Sulfur-Containing Heterocycles Derived by Reaction of ?-Keto Amides withLawesson's Reagent作者:Takehiko NishioDOI:10.1002/hlca.19980810531日期:——The reaction of ω-keto amides with Lawesson's reagent (LR: 2,4-bis(4-methoxyphenyl)-1,3,2,4-dithiadiphosphetane 2,4-disulfide) is described. Treatment of 3-keto amides (2-acylacetamides) 1 with LR gave the corresponding 3-keto thioamides 2, along with 1,2-dithiole-3-thiones 3. Treatment of 4-keto amides, 3-acyl propionamides 5, with LR yielded five-membered heterocycles, pyrroles 6 and/or 2-aminothiophenes
-
Alkynyl Fischer carbene complex as a traceless directing group for the regioselective cycloaddition of dithiolethiones to arylacetylene: synthesis of E-dithiafulvene thione and dithioesters作者:Alejandro M. Granados、Alejandro M. Fracaroli、Rita H. de Rossi、Pedro Fuertes、Tomás TorrobaDOI:10.1039/b715857b日期:——A phenylethynyl Fischer carbene complex was used as a traceless directing group for the regioselective cycloaddition of dithiolethiones to arylacetylene, which constitutes the first synthesis of E-dithiafulvene thione or dithioesters.
-
Immunomodulator 1,2-dithiol-3-thione derivative composition, use method申请人:Mitsui Toatsu Chemicals Inc.公开号:US04760078A1公开(公告)日:1988-07-26A novel 1,2-dithiol-3-thione derivative which has an immunomodulating property, which is expressed by the formula (I): ##STR1## wherein R denotes hydrogen, halogen, lower alkoxy group, lower alkyl group, amino group, lower alkyl-substituted amino group or lower alkoxycarbonyl group.
表征谱图
-
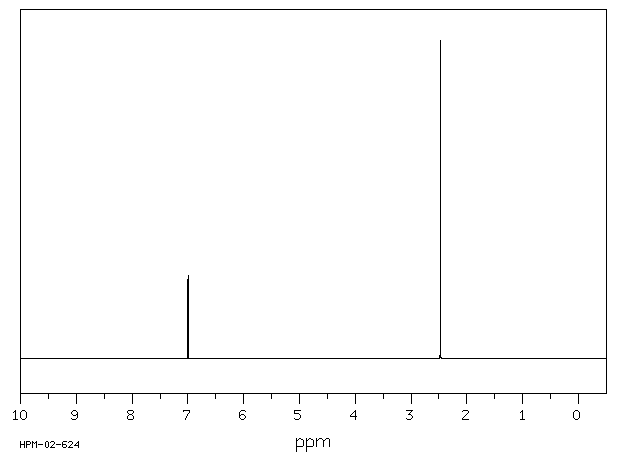
氢谱1HNMR
-
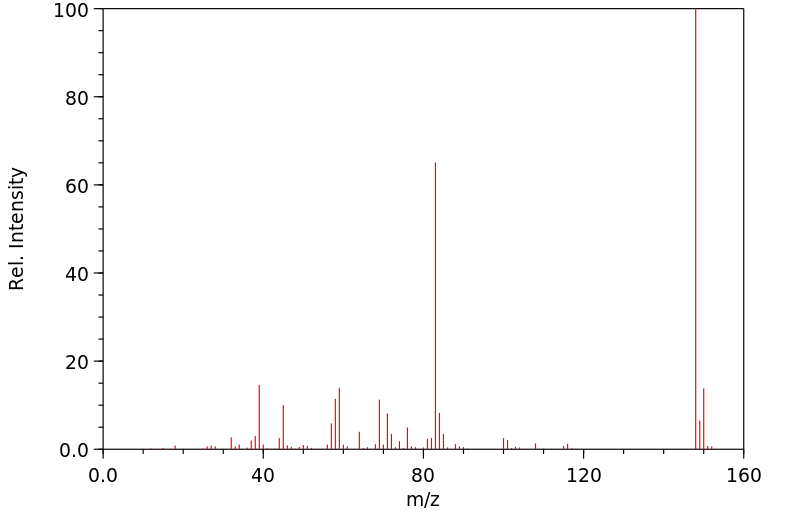
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
四硫杂富瓦烯-D4
四硫富瓦烯
四(戊硫代)四硫富瓦烯
四(十八烷基硫代)四硫富瓦烯
四(乙硫基)四硫富瓦烯[有机电子材料]
双(亚乙基二硫醇)四硫代富瓦烯
双(三亚甲基二硫代)四硫富瓦烯
三(四硫富瓦烯)双(四氟硼酸盐)复合物
[1,3]二噻唑并[4,5-d]-1,3-二噻唑,2,5-二(1,3-二硫醇-2-亚基)-
5-甲基二硫杂环戊烯-3-硫酮
5-氨基-3-硫代氧基-3H-(1,2)二硫杂环戊烯-4-羧酸乙酯
5-氨基-3-硫代氧基-3H-(1,2)二硫杂环戊烯-4-甲腈
5,6-二氢-4H-环戊并[1,2]二硫代-3-硫酮
4,4’,5-三甲基四硫富瓦烯
4-甲基二硫杂环戊烯-3-硫酮
4-新戊基-3H-1,2-二硫杂环戊烯-3-硫酮
4,5-二甲基-3H-1,2-二硫醇-3-酮
4,5,6,7-四氢苯并[1,2]二硫-3-硫酮
4,4’-二甲基连四硫富瓦烯
4,4,5,5,6,6,7,7-八氢二苯并四硫富瓦烯
3H-1,2-二硫杂环戊二烯-3-酮
3H-1,2-二硫杂环戊二烯-3-硫酮
2-(4,5-二甲基-1,3-二硫杂环戊烯-2-亚基)-4,5-二甲基-1,3-二硫杂环戊烯
2,3,6,7-四(2-氰乙基硫代)四硫富瓦烯
1,3-二噻唑,2-[4,5-二(癸基硫代)-1,3-二硫醇-2-亚基]-4,5-二(癸基硫代)-
1,3-二噻唑,2-[4,5-二(十四烷基硫代)-1,3-二硫醇-2-亚基]-4,5-二(十四烷基硫代)-
1,3-二噻唑,2-[4,5-二(十一烷基硫代)-1,3-二硫醇-2-亚基]-4,5-二(十一烷基硫代)-
(四甲基硫)四硫富瓦烯
3-[[2-[4,5-Bis(methylsulfanyl)-1,3-dithiol-2-ylidene]-5-[3-[[2-[4,5-bis(methylsulfanyl)-1,3-dithiol-2-ylidene]-5-[3-[[2-[4,5-bis(methylsulfanyl)-1,3-dithiol-2-ylidene]-5-[3-[[2-[4,5-bis(methylsulfanyl)-1,3-dithiol-2-ylidene]-5-(2-cyanoethylsulfanyl)-1,3-dithiol-4-yl]sulfanyl]propylsulfanyl]-1,3-dithiol-4-yl]sulfanyl]propylsulfanyl]-1,3-dithiol-4-yl]sulfanyl]propylsulfanyl]-1,3-dithiol-4-yl]sulfanyl]propanenitrile
4,5-Bis-{2-[2-(2-iodo-ethoxy)-ethoxy]-ethylsulfanyl}-4',5'-bis-methylsulfanyl-[2,2']bi[[1,3]dithiolylidene]
2-<4,5-bis(methylthio)-1,3-dithiol-2-ylidene>-5-(thiopyran-4-ylidene)-1,3,4,6-tetrathiapentalene
2,3-bis(2-cyanoethylthio)-6,7-bis(2-hydroxyethylthio)tetrathiafulvalene
4,5-bis(decylthio)-4'-(3-cyanopropyl)thio-5-methyltetrathiafulvalene
4,5,4',5'-Tetrakis-trimethylsilanylethynyl-[2,2']bi[[1,3]dithiolylidene]
bis(Dimethylvinylenedithio)tetrathiafulvalene
2,3-Bis{2-[2-(2-chloroethoxy)ethoxy]ethylthio}-6-(2-cyanoethylthio)-7-methylthiotetrathiafulvalene
3-[5-(2-Cyano-ethylselanyl)-2-methylsulfanyl-[1,3]dithiol-4-ylselanyl]-propionitrile
2-(4-Pent-4-ynyl-[1,3]dithiol-2-ylidene)-5,6-dihydro-[1,3]dithiolo[4,5-b][1,4]dithiine
2-(4-Nonadeca-4,6-diynyl-[1,3]dithiol-2-ylidene)-5,6-dihydro-[1,3]dithiolo[4,5-b][1,4]dithiine
5-Trifluoromethyl-[1,2]dithiole-3-thione
4-[(trimethylsilyl)ethynyl]-5-methyl-4',5'-ethylenedithiotetrathiafulvalene
[4-Methyl-5-methylsulfanyl-[1,2]dithiol-(3Z)-ylidene]-thioacetic acid S-methyl ester
1,3-Dithiolo[4,5-b][1,4]dithiin,5,6-dihydro-2-[4-(9-decynyl)-1,3-dithiol-2-ylidene]-
di(vinylthio)ethylenedithiotetrathiafulvalene
2,3:8,9-Bis(ethylendithio)-1,4,7,10-tetrathiafulvalen, CT-Komplex mit 2,5-Bis(cyanimino)-2,5-dihydro-3,6-diiodthieno<3,2-b>thiophen
4-ethyl-2-isopropylidene-[1,3]dithiole
2-[1-Chloro-1-methylsulfanylcarbonyl-meth-(Z)-ylidene]-5-methylsulfanyl-[1,3]dithiole-4-carbothioic acid S-methyl ester
tetra(vinylthio)tetrathiafulvalene