trans-2-Hydroxy-α-phenylzimtsaeure, (trans-2-Phenyl-3-<2-hydroxyphenyl>-acrylsaeure)
中文名称
——
中文别名
——
英文名称
trans-2-Hydroxy-α-phenylzimtsaeure, (trans-2-Phenyl-3-<2-hydroxyphenyl>-acrylsaeure)
英文别名
(E)-3-(2-hydroxyphenyl)-2-phenylprop-2-enoic acid
CAS
——
化学式
C15H12O3
mdl
——
分子量
240.258
InChiKey
KOWKQDIDRGLQDW-JLHYYAGUSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:18
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:57.5
-
氢给体数:2
-
氢受体数:3
上下游信息
反应信息
-
作为反应物:参考文献:名称:Synthesis and biological evaluation of some stilbene derivatives摘要:Several trans and cis stilbenes with substitution on the olefinic bridge were synthesized and characterized by IR, NMR and mass spectroscopy in an effort to obtain substances that could be more readily formulated. All the synthesized compounds were screened against Molt4/C8, CEM and L1210 cell lines. None of these compounds were endowed with pronounced cytostatic activity. However, Schiff derivatives emerged as cytostatic agents (IC(50): 0.77-10 mu g/ml) that deserve further investigation.DOI:10.1007/s00044-010-9484-1
-
作为产物:参考文献:名称:(E)-2,3-二苯基丙烯酸衍生物的抗氧化,抗酪氨酸酶和抗黑色素作用。摘要:在过去的十年中,我们在继续寻找强效美白剂的过程中,我们研究了许多含有常见(E)-β-苯基-α,β-不饱和羰基支架的酪氨酸酶抑制剂的功效,我们发现这些抑制剂对有效抑制蘑菇和哺乳动物酪氨酸酶。在这项研究中,我们探索了2,3-二苯基丙烯酸(2,3-DPA)衍生物的酪氨酸酶抑制作用,该衍生物也具有(E)-β-苯基-α,β-不饱和羰基基序。我们使用与苯乙酸和适当取代的苯甲醛的珀金反应,合成了十四种(E)-2,3-DPA衍生物1a-1n和一种(Z)-2,3-DPA衍生物1l'。在我们的蘑菇酪氨酸酶测定中,1c的酪氨酸酶抑制活性更高(76.43±3.53%,IC50 = 20.04±1.91 µM),而其他2种具有更高的抑制活性。3-DPA衍生物或曲酸(21.56±2.93%,IC50 = 30.64±1.27μM)。我们的蘑菇酪氨酸酶抑制结果得到我们的对接研究的支持,该研究表明化合物1c(-7.2 kcalDOI:10.1016/j.bmc.2019.04.020
文献信息
-
Synthesis and biological evaluation of cytotoxic properties of stilbene-based resveratrol analogs作者:Dênis Pires de Lima、Rodrigo Rotta、Adilson Beatriz、Maria Rita Marques、Raquel C. Montenegro、Marne C. Vasconcellos、Cláudia Pessoa、Manoel O. de Moraes、Letícia V. Costa-Lotufo、Alexandra Christine Helena Frankland Sawaya、Marcos Nogueira EberlinDOI:10.1016/j.ejmech.2008.05.003日期:2009.2deals with the preparation of stilbene-based resveratrol analogs by employing the Perkin reaction, aiming at synthesizing potential antitumor lead compounds and evaluating their pharmacological activities. The proliferation inhibitor test against tumor cell lines identified analogs 9 and 11 as the most active among all synthesized derivatives, presenting IC50 in micromolar range for certain cell lines
-
Stereospecificity in the Perkin–oglialoro reaction. The stereochemical configurations of some substituted α-phenylcinnamic acids作者:Malcolm Crawford、G. W. MooreDOI:10.1039/jr9550003445日期:——
-
Krishnaswamy et al., Indian Journal of Chemistry, 1964, vol. 2, p. 182,187作者:Krishnaswamy et al.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
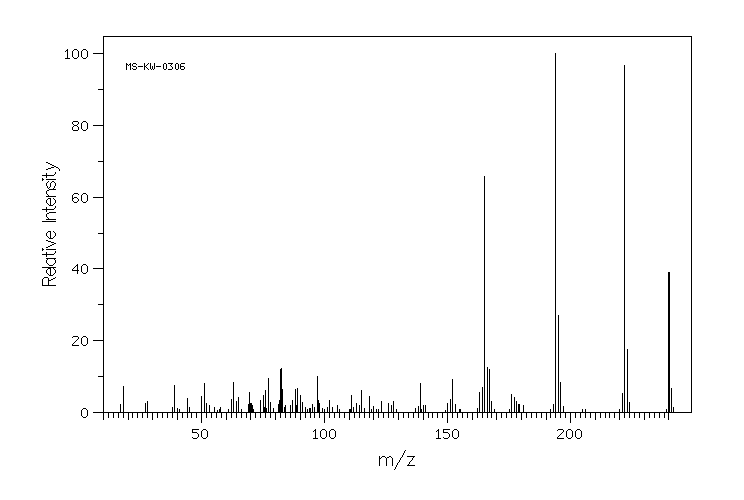
质谱MS
-
碳谱13CNMR
-
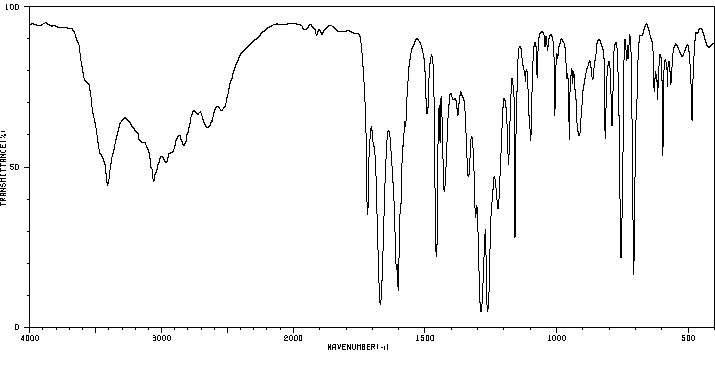
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(E,Z)-他莫昔芬N-β-D-葡糖醛酸
(E/Z)-他莫昔芬-d5
(4S,5R)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S,5R,5''R)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(4R,5S)-4,5-二苯基-1,2,3-恶噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4R,4''R,5S,5''S)-2,2''-(1-甲基亚乙基)双[4,5-二氢-4,5-二苯基恶唑]
(1R,2R)-2-(二苯基膦基)-1,2-二苯基乙胺
鼓槌石斛素
黄子囊素
高黄绿酸
顺式白藜芦醇三甲醚
顺式白藜芦醇
顺式己烯雌酚
顺式-白藜芦醇3-O-beta-D-葡糖苷酸
顺式-桑皮苷A
顺式-曲札芪苷
顺式-二苯乙烯
顺式-beta-羟基他莫昔芬
顺式-a-羟基他莫昔芬
顺式-3,4',5-三甲氧基-3'-羟基二苯乙烯
顺式-1-(3-甲基-2-萘基)-2-(2-萘基)乙烯
顺式-1,2-双(三甲基硅氧基)-1,2-双(4-溴苯基)环丙烷
顺式-1,2-二苯基环丁烷
顺-均二苯乙烯硼酸二乙醇胺酯
顺-4-硝基二苯乙烯
顺-1-异丙基-2,3-二苯基氮丙啶
非洲李(PRUNUSAFRICANA)树皮提取物
阿非昔芬
阿里可拉唑
阿那曲唑二聚体
阿托伐他汀环氧四氢呋喃
阿托伐他汀环氧乙烷杂质
阿托伐他汀环(氟苯基)钠盐杂质
阿托伐他汀环(氟苯基)烯丙基酯
阿托伐他汀杂质D
阿托伐他汀杂质94
阿托伐他汀杂质7
阿托伐他汀杂质5
阿托伐他汀内酰胺钠盐杂质
阿托伐他汀中间体M4
阿奈库碘铵
锌(II)(苯甲醛)(四苯基卟啉)
银松素
铜酸盐(5-),[m-[2-[2-[1-[4-[2-[4-[[4-[[4-[2-[4-[4-[2-[2-(羧基-kO)苯基]二氮烯基-kN1]-4,5-二氢-3-甲基-5-(羰基-kO)-1H-吡唑-1-基]-2-硫代苯基]乙烯基]-3-硫代苯基]氨基]-6-(苯基氨基)-1,3,5-三嗪-2-基]氨基]-2-硫代苯基]乙烯基]-3-硫代
铒(III) 离子载体 I
铀,二(二苯基甲酮)四碘-
钾钠2,2'-[(E)-1,2-乙烯二基]二[5-({4-苯胺基-6-[(2-羟基乙基)氨基]-1,3,5-三嗪-2-基}氨基)苯磺酸酯](1:1:1)
钠{4-[氧代(苯基)乙酰基]苯基}甲烷磺酸酯
钠;[2-甲氧基-5-[2-(3,4,5-三甲氧基苯基)乙基]苯基]硫酸盐
钠4-氨基二苯乙烯-2-磺酸酯