6-氨基十一烷 | 33788-00-0
中文名称
6-氨基十一烷
中文别名
——
英文名称
1-pentylhexylamine
英文别名
6-aminoundecane;6-undecylamine;undecan-6-amine;6-Undecanamin
CAS
33788-00-0
化学式
C11H25N
mdl
MFCD00047924
分子量
171.326
InChiKey
GFBRYGJZWXLRFR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:158°C/28mmHg(lit.)
-
密度:0.799±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:12
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:26
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:8
-
海关编码:2921199090
-
包装等级:II
-
危险类别:8
-
危险性防范说明:P501,P210,P264,P280,P370+P378,P303+P361+P353,P301+P330+P331,P363,P304+P340+P310,P305+P351+P338+P310,P403+P235,P405
-
危险品运输编号:2735
-
危险性描述:H314,H227
-
储存条件:| 室温 |
SDS
反应信息
-
作为反应物:参考文献:名称:萘酰亚胺作为基于NiO的p型染料敏化太阳能电池的新型p型敏化剂摘要:我们报告两种用于p型染料敏化太阳能电池的有效材料,即S64和S85。在这项研究中提出了用于NiO基染料敏化太阳能电池的新型p型光敏剂。使用红外,核磁共振和质谱技术已成功合成并表征了两种化合物,该化合物包含附加到1,8-萘酰亚胺发色团上的二苯胺供体。此外,使用吸收光谱,发射光谱和循环伏安法研究了它们的电化学性质。将羧酸锚定基团添加到两种萘化合物中,从而使染料吸附到染料的NiO电极上。这些新材料在具有扩展π共轭和长烷基链的有机溶剂中具有出色的溶解性。这些染料在基于NiO的p型染料敏化太阳能电池中显示出良好的效率。另外,DOI:10.1039/d0nj03266b
-
作为产物:参考文献:名称:荧光N-取代的苯并per萘“燕尾”单酰亚胺的合成,表征和光物理研究摘要:合成了一组N-取代的苯并per单酰亚胺(BPI)荧光团,并对其结构和光物理性质进行了表征。在“燕尾”烷基胺存在下缩合苯并per-1,2-二羧酸酐产生的荧光团可溶于多种有机溶剂,在近紫外光下高度吸收(ε334 = 79 000 M –1 cm –1),并且在可见光范围内发出荧光。使用稳态和与时间相关的单光子计数研究了化合物的光物理行为。合成的BPI表现出正的发色光发射(λem(己烷)= 469 nm; λem(乙醇)= 550 nm)作为溶剂极性的函数,在所研究的极性范围内,其激发态寿命(9.6–6.5 ns)和荧光量子产率(0.27–0.44)几乎没有变化。使用Lippert-Mataga方法分析了色度变化。在非极性烃类溶剂中,观察到紧密间隔(562 cm –1)的S 1和S 2激发态双重发射的证据。初步确定了异常S 2发射的峰值。DOI:10.1021/jo200529p
文献信息
-
DIARYLMETHYLAMIDE DERIVATIVE HAVING ANTAGONISTIC ACTIVITY ON MELANIN-CONCENTRATING HORMONE RECEPTOR申请人:Banyu Pharmaceutical Co., Ltd.公开号:EP2272841A1公开(公告)日:2011-01-12[Problem] To provide a melanin-concentrating hormone receptor antagonist useful as a pharmaceutical agent for central diseases, circulatory diseases, and metabolic diseases. [Means for Resolution] Provided is a diarylmethylamide derivative represented by formula (I): Wherein R1a, R1b, R2a, R2b, R3a, and R3b independently represent a hydrogen atom or the like, R4 represents a hydrogen atom, C1-6 alkyl, or the like, R5 represents a hydrogen atom or the like, Z represents C1-6 alkyl or the like, or R4 and Z together form a 4- to 6-membered nitrogen-containing hetero ring, Y1 represents H or the like, Y2 represents H, or Y1 and Y2 together form - O-CH2-, W represents C, SO, or the like, Ar1 represents 6-membered aryl or the like, Ar2 represents 6-membered aryl or the like, and ring A represents a benzene ring, a pyridine ring, or the like.
-
Substituted 4-Hydroxypyrimidine-5-Carboxamides
-
[EN] DIARYLMETHYLAMIDE DERIVATIVE HAVING MELANIN-CONCENTRATING HORMONE RECEPTOR ANTAGONISM<br/>[FR] DÉRIVÉ DE DIARYLMÉTHYLAMIDE PRÉSENTANT UNE ACTIVITÉ D'ANTAGONISTE DES RÉCEPTEURS DE L'HORMONE DE MÉLANO-CONCENTRATION申请人:MERCK SHARP & DOHME公开号:WO2011037771A1公开(公告)日:2011-03-31The present invention is directed to diarylmethylamide derivatives represented of structural formula I which are melanin-concentrating hormone receptor antagonists, and are useful as an agent for the prevention, treatment, or remedy of various circulatory diseases, neurological diseases, metabolic diseases, reproductive system diseases, respiratory diseases, digestive diseases, and the like. The invention is also directed to pharmaceutical compositions comprising these compounds and the use of these compounds and compositions in the prevention or treatment of such diseases in which the melanin-concentrating hormone is involved. I
-
Novel NIR-absorbing conjugated polymers for efficient polymer solar cells: effect of alkyl chain length on device performance作者:Wei Yue、Yun Zhao、Shuyan Shao、Hongkun Tian、Zhiyuan Xie、Yanhou Geng、Fosong WangDOI:10.1039/b818885h日期:——Three low bandgap conjugated polymers, i.e., PDTPBT-C8, PDTPBT-C6 and PDTPBT-C5, which consist of alternating N-alkyl dithieno[3,2-b:2′,3′-d]pyrrole and 2,1,3-benzothiadiazole units and carry 1-octylnonyl, 1-hexylheptyl and 1-pentylhexyl as side chains, respectively, were synthesized. These polymers show strong absorption in the wavelength range of 600–900 nm with enhanced absorption coefficient as the length of alkyl chain decreases. The film morphology of the polymers and 1-(3-methoxycarbonyl)propyl-1-phenyl-[6,6]-C-61 (PCBM) blends is also dependent on the alkyl chain length. As the length decreases, the film becomes more uniform and the domian size decreases from 400–900 nm for PDTPBT-C8 to ∼50 nm for PDTPBT-C5. Bulk heterojunction photovoltaic solar cells (PSCs) were fabricated based on the blend of the polymers and PCBM with a weight ratio of 1:3. The device performance is dramatically improved as the length of the side chain decreases, due to enhanced film absorption coefficient and improved film morphology. With the polymer PDTPBT-C5, which carries the shortest alkyl chain, power conversion efficiency (PCE) up to 2.80% has been achieved. This result indicates that optimizing the structure of the solublizing alkyl chain is also crucial for the design and synthesis of high performance PSC polymeric materials.合成了三种窄带隙共轭聚合物,即PDTPBT-C8,PDTPBT-C6和PDTPBT-C5,它们由交替的N-烷基二噻吩并[3,2-b:2',3'-d]吡咯和2,1,3-苯并噻二唑单元组成,并分别带有1-辛基壬基,1-己基庚基和1-戊基己基侧链。这些聚合物在600-900 nm波长范围内显示出强烈的吸收,并且随着烷基链长度的减少,吸收系数增强。聚合物和1-(3-甲氧羰基)丙基-1-苯基-[6,6]-C-61(PCBM)混合物的膜形态也取决于烷基链的长度。随着链长的减少,膜变得更加均匀,域尺寸从PDTPBT-C8的400-900 nm减小到PDTPBT-C5的约50 nm。基于聚合物和PCBM的混合物(重量比为1:3)制备了体异质结光伏太阳能电池(PSCs)。由于膜吸收系数的增强和膜形态的改善,随着侧链长度的减少,器件性能显著提高。带有最短烷基链的聚合物PDTPBT-C5,其功率转换效率(PCE)达到了2.80%。这一结果表明,优化溶解性烷基链的结构对于高性能PSCs聚合物材料的设计和合成也至关重要。
-
Synthesis and photovoltaic properties of thiophene–imide-fused thiophene alternating copolymers with different alkyl side chains作者:Tomokazu Umeyama、Masaaki Oodoi、Osamu Yoshikawa、Takashi Sagawa、Susumu Yoshikawa、Douvogianni Evgenia、Noriyasu Tezuka、Yoshihiro Matano、Kati Stranius、Nikolai V. Tkachencko、Helge Lemmetyinen、Hiroshi ImahoriDOI:10.1039/c1jm11531f日期:——A series of novel polythiophene derivatives comprised of alternating structures of N-alkylated thieno[3,4-c]pyrrole-4,6-dione (nTPD, n = number of carbon atoms from 11 to 18 in alkyl chains) and pristine thiophene were synthesized by Stille coupling reaction between 2,5-dibromo- or 2,5-diiodo-nTPD and 2,5-bis(tributylstannyl)thiophene. The effect of alkyl side-chain structures at the N-atom of TPD on the optical, electrochemical, and photovoltaic properties has been investigated systematically. In addition, these properties were compared with a widely used polythiophene derivative, i.e., poly(3-hexylthiophene) (P3HT). Optical bandgaps of the obtained polymers PnTPDTs estimated from absorption and fluorescence spectra were 1.8â2.1 eV, which were comparable or smaller than that of P3HT (2.0 eV). HOMO and LUMO energy levels of PnTPDTs were determined by electrochemical and optical measurements. The HOMO levels are â5.4 to â5.7 eV, which are lower than that of P3HT (â5.2 eV). The photovoltaic properties of the devices consisting of PnTPDT (n = 11, 12, 13, 18) with [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) were investigated. The short-circuit currents (JSC) in the PnTPDT:PCBM devices were relatively low and varied significantly with alkyl side-chain structures (0.81â2.29 mA cmâ2), whereas the open-circuit voltage (VOC) values were higher by 0.1â0.2 V than that in the P3HT:PCBM device. The P12TPDT:PCBM device exhibited the photocurrent generation exceeding wavelength of 750 nm, but the power conversion efficiency was found to be moderate (0.75%).一系列新颖的聚噻吩衍生物,由N-烷基化的噻吩并[3,4-c]吡咯-4,6-二酮(nTPD,其中n代表烷基链中的碳原子数,范围从11到18)和原始噻吩的交替结构组成,通过Stille偶联反应合成了2,5-二溴或2,5-二碘的nTPD与2,5-双(三丁基锡)噻吩的反应。系统研究了TPD的N原子上烷基侧链结构对光学、电化学和光伏性能的影响。此外,这些性能与广泛使用的聚噻吩衍生物,即聚(3-己基噻吩)(P3HT)进行了比较。从吸收和荧光光谱估计得到的聚合物PnTPDTs的光学带隙为1.8至2.1 eV,与P3HT(2.0 eV)相当或更小。通过电化学和光学测量确定了PnTPDTs的HOMO和LUMO能级。HOMO能级为-5.4至-5.7 eV,低于P3HT(-5.2 eV)。研究了由PnTPDT(n=11, 12, 13, 18)与[6,6]-苯基-C61-丁酸甲酯(PCBM)组成的器件的光伏性能。PnTPDT:PCBM器件中的短路电流(JSC)相对较低,并且随烷基侧链结构的显著变化(0.81至2.29 mA cm-2),而开路电压(VOC)值比P3HT:PCBM器件高0.1至0.2 V。P12TPDT:PCBM器件表现出超过750 nm波长的光电流生成,但功率转换效率中等(0.75%)。
表征谱图
-
氢谱1HNMR
-
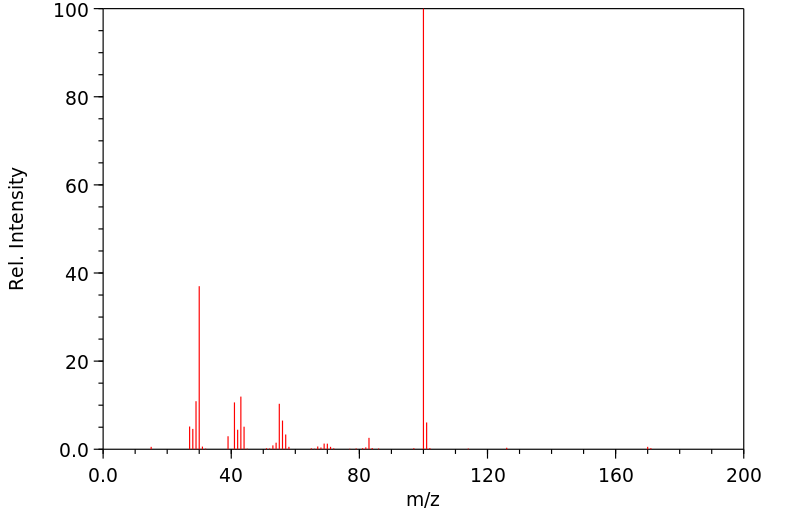
质谱MS
-
碳谱13CNMR
-
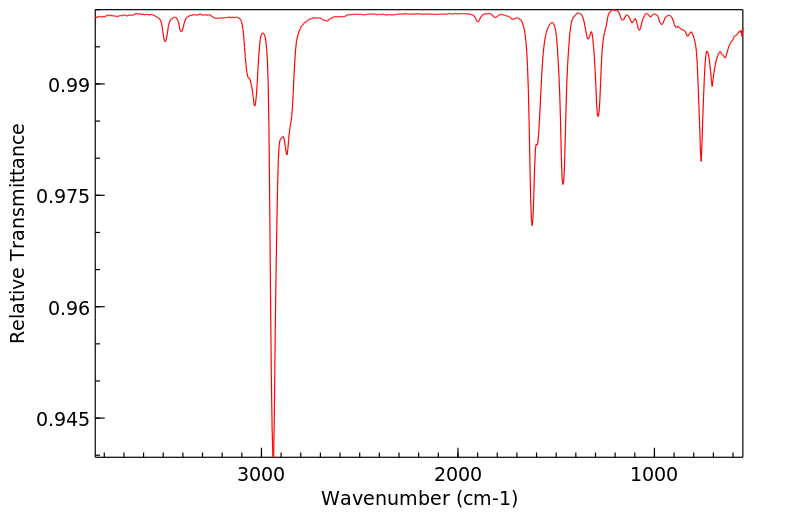
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷